
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Nifn statement on domperidone

Statement by The National Infant Feeding Network: December 2014
The use of Domperidone in inadequate lactation
The purpose of this paper is to review and clarify the unlicensed use of Domperidone as a galactogogue, following the statements from the EMA and MHRA in April 2014 (1, 2).
A recent Europe-wide review has recommended updates to the treatment advice for the licensed use of Domperidone,
following evaluation of the benefits and risks. The review was triggered following continued reports of cardiac side ef-fects, and a small increased risk of serious cardiac side effects was confirmed.
A higher risk was observed in:
Patients older than 60 years
Adults taking daily oral doses of more than 30mg
Those taking QT-prolonging medicines or CYP3A4 inhibitors concomitantly (E.g. Erythromycin, Sertraline, Keto-conazole, Salbutamol)
Its licensed use is now restricted to relief of nausea and vomiting and the dose and duration of use have been reduced. It is now contraindicated in those with underlying cardiac conditions and other risk factors.
Although the EMA stated that the scope of the review did not cover use outside the licensed indications (off-label use) it did add that the principles behind these recommendations should be considered whenever Domperidone is used.
Rationale for the use of Domperidone as a galactogogue
Domperidone is a dopamine antagonist. It works by blocking the receptors for the neurotransmitter dopamine in the gut and in the part of the brain linked to vomiting. Specifically it works on peripheral dopamine receptors in the GI wall and
CTZ centre of the brainstem. Prolactin levels are increased as a result of blocking the dopamine receptors.
Unlike Metoclopramide, and most other dopamine antagonists, Domperidone passes poorly into the brain and has thus has few central nervous system side effects, such as depression. Domperidone has a higher molecular weight and binds more strongly to plasma proteins than Metochlopramide, and is thus less likely to enter breastmilk.
Evidence of effectiveness as a galactogogue
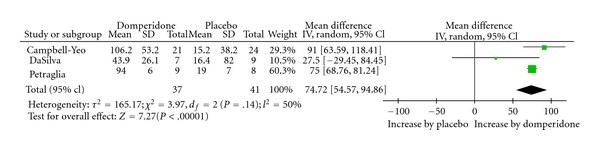
Osadchy (2012) conducted a Systematic Review and Meta-Analysis of RCTs which had been conducted to examine the
effect of Domperidone on insufficient lactation in postpartum women (3). Three RCTs met inclusion criteria (78 partici-pants), and all showed statistically significant increases in breast milk production following treatment with Domperi-done. Analysis of pooled data demonstrated a relative increase of 74.72% in daily milk production with Domperidone
treatment compared to placebo.
A network of infant feeding specialists promoting and sharing evidence-based practice to health professionals across England.

How much for how long?
General criteria for the administration of any drug, for any reason, would be to use the lowest (effective) dose for the
shortest (effective) period of time. This is re-iterated in the EMA / MHRA recommendations.
In the case of Domperidone used as a galactogogue, the trial evidence suggests that this would be 10 mgs, three times per day (30mgs per 24 hours). This is compatible with the EMA / MHRA recommendations.
Duration of use for nausea and vomiting
Following the EMA / MHRA recommendations, the indications for use are now restricted to nausea and vomiting. It is thus no longer recommended for bloating and heartburn in adults, nor for treating reflux in babies. (However it can still be given to babies to control vomiting.)
For its licensed use, the EMA states that the maximum treatment duration should not usually exceed one week. This is re-peated on the MRHA website (2).
However in the Department of Health letter sent to health professionals, the word "usually" has been omitted (4).
Nausea and vomiting are symptoms of pathology. The person for whom Domperidone is prescribed for this reason may be ill (e.g. suffering migraine) or be suffering as a result of receiving other medication for an illness (e.g. chemotherapy). In the case of pathology it might be expected that treatment would be reviewed within a week (or less) of the symptoms developing, particularly if they were not resolving.
Duration of use as a galactogogue
Data from the trials reviewed by Osadchy (2012) suggest that continuing treatment beyond a week, up to 14 days, makes a
difference to milk yield.
Time of measurement
Daily milk yield
Experimental: control
Petralgia et al.
2nd treatment day
6th treatment day
10th treatment day
2nd treatment day
6th treatment day
7th treatment day
1st treatment day
14th treatment day
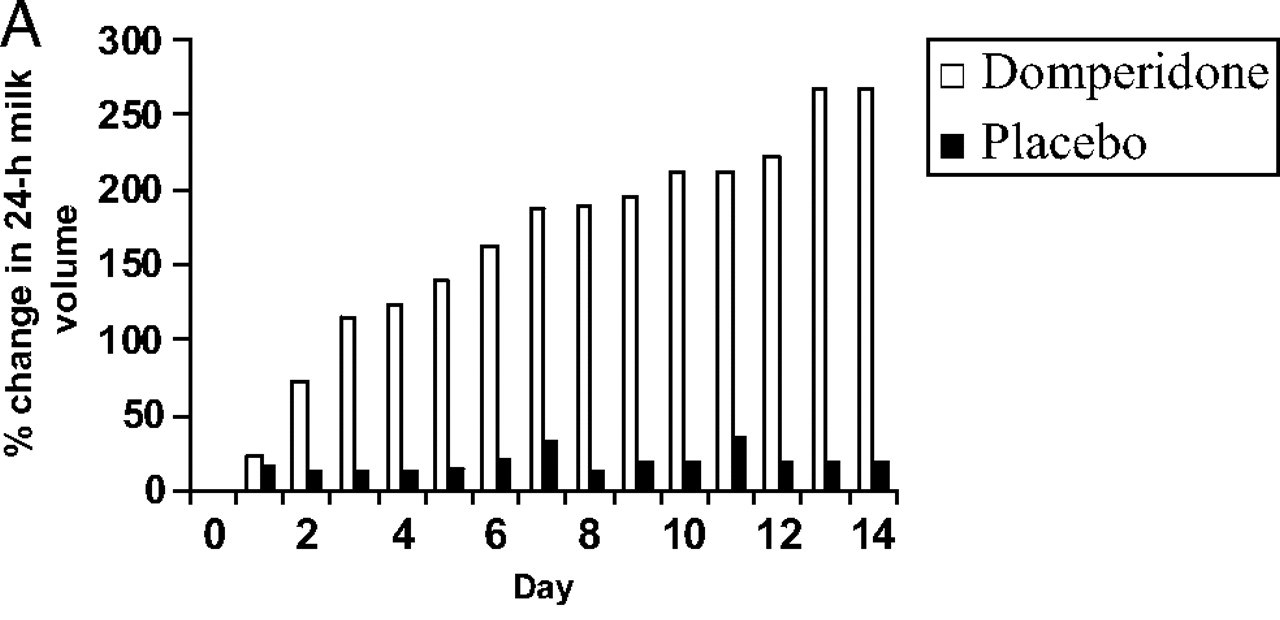
The mean increase in breast milk volume in the Domperidone group was 106% ([380 - 184] x 100/184) and in the placebo group it was 15% ([250 -218] x 100/218).
See table below*
*Mean within-subject percent change in 24-hour breast milk volume in the domperidone and placebo groups from day 0 to day 14 after initiation of treatment Campbell-Yeo et al. (2010)
A network of infant feeding specialists promoting and sharing evidence-based practice to health professionals across England.

Effect of Domperidone on the Composition of Preterm Human Breast Milk (Courtesy of Campbell-Yeo et al, 13)
Women for whom Domperidone is prescribed as a galactogogue are not ill; they will be healthy young mothers for whom there are no contra-indications.
They will also be mothers for whom other methods of increasing milk supply (often for a pre-term baby) will have been unsuccessful. It would seem logical to prescribe the drug at the dose and for the duration supported by the available evi-dence.
Midlands Medicines, the UK Drugs in Lactation Advisory Service (formerly UKMI) has, from 2006, offered guidance on the
use of Domperidone in inadequate lactation in the form of a Q&A sheet on its website (5). The most recent update (2012) offered the following as a summary:
A health professional should always be involved in the decision to use a galactogogue.
Drugs to manage inadequate lactation should only be used where there is objective evidence to support diagnosis and where non-drug methods have failed
There are no drugs licensed in the UK to improve lactation.
Domperidone is considered to be the agent of choice for inadequate lactation because of its superior side effect profile, efficacy, and minimal passage into breast milk.
Domperidone should not be used for inadequate lactation where the mother or infant has a cardiac disorder or are receiving treatment with drugs known to affect the QT interval e.g. ketoconazole or erythromycin, in which case metoclopramide is preferred.
A maternal daily dose of 30mg Domperidone should not be exceeded.
Further studies are needed to determine the optimum regimen and duration of treatment.
There are insufficient data to support the use of herbal remedies.
This document reviewed the trials included in the Osadchy meta-analysis among many others, but did not give guidance on the optimum duration of treatment. (See above).
A network of infant feeding specialists promoting and sharing evidence-based practice to health professionals across England.

Following the EMA statement the following update has been added to the website (6):
"The use of Domperidone to enhance lactation is not specifically covered by the review as it is an unlicensed (off-label) indi-
cation. However, the recommendations from the review should apply to its use as a galactogogue."
"As there are limited alternative options for the stimulation of lactation, the use of Domperidone can be considered provided non-pharmacological options have been unsuccessful.
A maternal dose of 30mg daily for a maximum of 1 week should not be exceeded.
Domperidone should not be used if the mother or infant:
Have conditions where cardiac conduction is, or could be, impaired.
Are receiving other medications known to prolong QT or potent CYP3A4 inhibitors.
have severe hepatic impairment.
If treatment is still required after a week, metoclopramide can be used for up to five days."
(There is currently no link, and no reference, to support the final statement.)
The 2012 Osadchy paper (3) concluded that the consistency of the Domperidone effect across the studies (necessarily in
the dosage and for the duration used in the studies) enhances the confidence of its beneficial effect as a galactogogue.
No maternal or neonatal adverse events were observed in any of the three trials.
The available data indicates minimal excretion of Domperidone into breast milk with extremely low (less than 0.01% of the maternal weight-adjusted dose) infant exposure via breast milk and no side effects have been reported in exposed
infants [7–10].
The American Academy of Pediatrics lists Domperidone as compatible with breastfeeding [11]. Furthermore, a 2012 con-sensus statement by Canadian breastfeeding experts, (which included, amongst others, Daniel Flanders, Aviva Lowe, Mi-
chael Kramer, Orlando da Silva, Carole Dobrich, Marsha Campbell-Yeo, Edith Kernerman and Jack Newman) concluded that there is no evidence of increased risk to health young women who do not fall into any of the at risk categories (12). In the realm of clinical practice, where the balance between desirable and undesirable effects often guides treatment de-cisions, the current analysis supports consideration that Domperidone might be an effective treatment option for select-ed women with inadequate lactation.
Many GPs and hospital doctors are well acquainted with the off-label use of medicines in their practice; for example
drugs that are only licensed for use to treat epilepsy are frequently prescribed to treat neuropathic pain. In these cases the prescribed dose and duration of the medication may be different from that for the licensed use. In these situations the prescribers take the responsibility for prescribing off-label because they feel the evidence base supports it.
It remains to be seen if this will continue to be the case for Domperidone.
A network of infant feeding specialists promoting and sharing evidence-based practice to health professionals across England.

In Summary
Domperidone should only be considered when all other methods and support for increasing breastmilk are in place.
A suitably qualified health professional should always be involved in the decision.
Domperidone should not be used where the mother or infant has a cardiac disorder or are receiving treatment with drugs known to affect the QT interval.
A maternal daily dose of 30mg Domperidone should not be exceeded.
For the purpose of increasing milk supply, the evidence supports the use of 10mgs, three times a day, for 10-14 days.
Ongoing monitoring and support should continue whilst Domperidone is in use and thereafter as necessary.
References
Osadchy A. Myla E. Moretti. & Koren G. (2012) Effect of domperidone on insufficient lactation in puerperal women: A systematic review and meta-analysis of randomized controlled trials. Obstetrics and Gynecology International Vol 2012, Article ID 642893
Hofmeyer GJ, Van Iddekinge B. (1983) Domperidone and lactation. The Lancet. 1(8325):p. 647.
Hofmeyr GJ, Van Iddekinge B, Blott JA. (1985) Domperidone: secretion in breast milk and effect on puerperal prolactin levels. British Journal of Obstetrics and Gynaecology. 92(2):141–144.
Da Silva OP, Knoppert DC, Angelini MM, Forret PA. (2001) Effect of domperidone on milk production in mothers of
premature newborns: a randomized, double-blind, placebo-controlled trial. Canadian Medical Association. 164(1):17–21
Wan EWX, Davey K, Page-Sharp M, Hartmann PE, Simmer K, Ilett KF. (2008) Dose-effect study of Domperidone as a
galactagogue in preterm mothers with insufficient milk supply, and its transfer into milk. British Journal of Clinical Pharmacology. 66(2):283–335.
Wright N. (2011) Domperidone for improving breastmilk produc-tion
Consensus statement by breastfeeding experts on Health Canada advice. May 2012.
Campbell-Yeo ML, Johnston CC, Joseph KS, et al (2010). Effect of domperione on the composition of preterm hu-
man breastmilk. Pediatrics. 125(1): 107-14.
A network of infant feeding specialists promoting and sharing evidence-based practice to health professionals across England.
Source: http://www.unicef.org.uk/Documents/Baby_Friendly/Networks/NIFN_domperidone_2015.pdf
SPINAL CORD MEDICINEEDUCATIONAL MATERIALS FOR PATIENT AND FAMILY BLADDER MANAGEMENT FOLLOWING SPINAL CORD INJURY/IMPAIRMENT Frazier Rehab Institute DISCLAIMERThe information contained herein is intended to be used in accordance with the treatment plan prescribed by your physician and with the prior approval of your physician. You should not begin using any of the information and/or methods described in these publications until you have consulted your physician. Jewish Hospital & St. Mary's HealthCare, Inc. D.B.A. Frazier Rehab Institute, its affiliates, associates, successors and assigns, as well as its trustees, officers, directors, agents and employees are not liable for any damages resulting from the use of this publication.
Important InformatIon on health and medIcal care IMPORTANT: If you are a citizen of the EU/EEA and can present a valid European health insurance card (EU-card) or are nationally registered in Sweden you are covered by the healthcare offered by the County Council of Västerbotten. You might also be covered by this healthcare if you are a non EU/EEA citizen. You must then have a residen-ce permit for more than a year and be nationally registered in Sweden. The











