
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Viapost.nu
Clinical Chemistry 57:4
Automation and Analytical Techniques
Measurement of Hemoglobin A
from Filter Papers for
David A. Egier,1 Judy L. Keys,1 S. Kim Hall,1 and Matthew J. McQueen1,2,3*
BACKGROUND: Stability and transport challenges make
standardized protocols, and analyses were performed
hemoglobin (Hb) A1c measurement from EDTA whole
in an NGSP-certified laboratory, supporting the use of
blood (WB) inconvenient and costly for large-scale
FP collection cards in large multinational studies.
population studies. This study investigated Hb A1c
2011 American Association for Clinical Chemistry
measurement from WB blotted on filter paper (FP) in aLevel I National Glycohemoglobin StandardizationProgram (NGSP)-accredited laboratory.
Diabetes affects ⬎285 million people globally (112%
METHODS: Three Bio-Rad Variant™ II HPLC instru-
increase since 1995); and this number is projected to
ments and WB and FP specimens were used. Precision,
increase to almost 440 million by 2025
(1, 2 ). Correla-
accuracy, linearity, and readable total area of the 6.5-
tion between hyperglycemia and complications such as
min (-thalassemia method) Variant II HbA
retinopathy and neuropathy was established by the Di-
Dual Program were assessed. Hb A
abetes Control and Complications Trial (DCCT)4
measured using in-house FP QC samples. The
(1983–1993)
(3, 4 ), and the cardiovascular disease re-
INTERHEART (a study of the effect of potentially
lationship was established by the Epidemiology of Di-
modifiable risk factors associated with myocardial
abetes Interventions and Complications study (1993
infarction in 52 countries) and CURE (Clopidogrel
onward)
(5 ). Because these complications are the lead-
in Unstable Angina to Prevent Recurrent Events)
ing causes of morbidity and mortality in people with
studies provided chromatographs for morphometric
diabetes and are reduced when hemoglobin A1c (Hb
analyses and interoperator variability experiments.
A1c) is ⬍7%, stringent glycemic monitoring and con-
Statistical analyses were performed to assess long-
trol is essential
(6 ). Hb A1c measurement is used with
term sample stability, WB vs FP agreement, and sig-
other glucose tests in screening for diabetes
(7 ), and
nificance of Hb A1c peak integration.
Hb A1c monitoring influences clinical treatmentdecisions.
RESULTS: Intra- and interassay CVs were ⱕ2.00%. Total
area counts between 0.8 and 5.5 ⫻ 106 V/s produced
1c is the amino-terminal nonenzymatic gly-
cation (on amino-terminal valine residues of the
accurate Hb A1c results. The regression equation for
-chain) product of Hb A and depends on the concen-
agreement between WB(
x) and FP(
y) was as follows:
tration of blood glucose and the lifespan of circulating
y ⫽ 0.933
x ⫹ 0.4 (n ⫽ 85). FP QC samples stored at
red blood cells (approximately 120 days)
(8, 9 ). Hb A
70 °C and tested over approximately 3 years yielded
levels (expressed as a percentage of total Hb A) reflect
CVs of 1.72%–2.73% and regression equations with
long-term blood glucose concentrations and thus the
slopes of ⫺1.08 ⫻ 10⫺4 to 7.81 ⫻ 10⫺4. The CURE
efficacy of glycemic control
(10, 11 ) over the prior 2–3
study, with better preanalytical preparation, achieveda 97% reportable rate, and the reportable rate of the
months, 50% of which is representative of the previous
INTERHEART study was 85%.
month, 25% of the previous 2 months, and 25% of theprevious 3 months
(12, 13 ).
CONCLUSIONS: The FP collection method described pro-
Whole blood (WB) venous samples collected by
vided accurate, robust, and reproducible measurement
venipuncture into EDTA Vacutainer Tubes are used
most commonly for Hb A
1c using the Bio-Rad Variant II HPLC autoana-
1c measurement, and trans-
lyzer when FP specimens were prepared according to
portation to a central laboratory in large-scale
1 Clinical Research and Clinical Trials Laboratory (CRCTL), Hamilton General
Received September 18, 2010; accepted January 12, 2011.
Hospital, Hamilton, Ontario, Canada; 2 Population Health Research Institute,
Previously published online at DOI: 10.1373/clinchem.2010.156380
Hamilton Health Sciences, Hamilton, Ontario, Canada; 3 Department of Pathol-
4 Nonstandard abbreviations: DCCT, Diabetes Control and Complications Trial;
ogy and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada.
Hb A1c, hemoglobin A1c; WB, whole blood; FP, filter paper; CRCTL, Clinical
* Address correspondence to this author at: Hamilton General Hospital, 237
Research and Clinical Trials Laboratory; NGSP, National Glycohemoglobin
Barton St. East, Hamilton, Ontario, Canada L8L 2X2. Fax 905-577-1476; e-mail
Standardization Program; CURE, Clopidogrel in Unstable Angina to Prevent
Recurrent Events; LA1c, labile Hb A1c.
Table 1. Criteria used to assess chromatograph acceptability.
⬍800 000 V/s
⬎5 500 000 V/s
integration peak does not properly integrate area under elution peak
Inadequate separation
Inadequately separated LA
No integration peak present for Hb A
Blank chromatograph
No elution pattern present
Another peak ⬎ Hb A peak
Hb A peak must be the largest
Variant/unknown peak interference
Large variant/unknown peak present at retention time similar to that of Hb
and interferes with Hb A
Abnormal baseline curvature
Dramatic changes in baseline curvature
Software exception
Error code; Bio-Rad Clinical Data Management software does not properly
population-based studies is costly. The stability of Hb
ponentially modified gaussian algorithm to calculate
1c is questionable when there are variable and ex-
2, Hb F, and Hb A1c.
tended periods between collection and analysis
Hb A1c was measured from WB and from drops of
(14, 15 ). Blood sample collection onto filter paper
the same WB applied to FP. The Variant II instruments
(FP) has been implemented in epidemiologic studies
were calibrated daily at the beginning of the first ana-
(16 ) and significantly decreases transportation costs
lytical run with dual-level (-Thal CalSet; Bio-Rad)
and limits the challenges of shipping dangerous
standardized to the DCCT. Bio-Rad A2/F controls were
goods in large-scale multinational population stud-
tested at the beginning of each run, and in-house FP
ies. Previous work showed that Hb A
QC samples (blotted from WB and stored at ⫺70 °C)
are stable and provide reliable, reproducible values
were tested at the beginning and end of each analytical
after 5–7 days at room temperature, 10 days at
run. The FP and WB samples and Bio-Rad A2/F con-
4 – 6 °C, and several months at ⫺70 °C
(17–20 ). This
trols were prediluted in 1 mL Bio-Rad hemolyzing buf-
investigation examined FP collected in 78 countries
fer. A 3/16-inch disk was punched from each FP sample
representing every region of the world for suitability
into the extraction buffer; 30 min was allowed for
blood to elute into solution at room temperature,
which was followed by removal of the disk. Five micro-
Materials and Methods
liters of each WB sample and Bio-Rad control wasadded to 1 mL of hemolyzing buffer. Vials were in-
ANALYTICAL INSTRUMENTS AND Hb A
verted to mix thoroughly before analysis. Each result-
Three Bio-Rad Variant II ion-
ing chromatograph was initially screened by using ac-
exchange HPLC instruments were used with the 6.5-
ceptance/repeat/rejection criteria developed within the
min (-thalassemia method) Variant II HbA
Clinical Research and Clinical Trials Laboratory
Dual Program to measure Hb A
(CRCTL) (Table 1).
1c in WB and extracted
FP samples. Prior investigation using the 1.5-min(Turbo) and 3-min Variant II programs resulted in in-
sufficiently separated chromatograph elution peaks.
Intraassay precision. Intraassay precision was deter-
The instrument autoinjects samples into an analytical
mined by using in-house FP samples stored at ⫺70 °C
cartridge, which separates hemoglobins on the basis of
(normal approximately 5.6% and high approximately
ionic interaction with the cartridge material. Eluted he-
8.0%). Ten FP hemolysates of each level were prepared
moglobins pass through the detection station, where
and analyzed on each of 3 instruments on each of 3
changes in absorbance are read at 415 nm (background
days. Precision was determined as CV%: [(SD/
corrected at 690 nm). Clinical data management soft-
mean) ⫻ 100], calculated from the mean and SD of
ware analyzes the raw absorbance data and uses an ex-
each sample on each instrument each day.
Clinical Chemistry 57:4 (2011)
Measurement from Filter Papers in Population Studies
Interassay precision. Interassay precision was assessed
and matching WB sample were analyzed on the same
by using freshly prepared Bio-Rad WB control sam-
run. Passing–Bablok and Bland–Altman method com-
ples, in-house FP QC samples (normal approximately
parison analyses were performed to evaluate bias be-
5.6% and high approximately 9.0%), in-house FP sam-
tween sample types.
ples (normal approximately 5.6% and high approxi-mately 8.0%), and 5 WB samples (WB1–5), tested as
FP STABILITY
WB and WB blotted on FP (in-house FP QC samples
FP QC samples prepared in-house in the normal (ap-
and WB1–5 samples stored at ⫺70 °C for 1 year and 8
proximately 5.6% and 5.3%) and high (approximately
months, respectively). Hemolysates were prepared
9.0% and 9.7%) Hb A1c range were stored at ⫺70 °C
daily and tested on each of 3 days. FP samples,
and tested at the beginning and end of every analytical
WB1–5 samples, and Bio-Rad WB controls were
run over approximately 3 years. Sample stability was
tested twice daily; in-house FP QC samples and
assessed by using Deming linear regression and calcu-
WB1–5 FP samples were tested once daily on each
lated as the CV% of all measures on each instrument
instrument for 3 days. The mean, SD, and CV% for
for each QC.
each specimen type were calculated across all instru-ments over 3 days.
STATISTICAL METHODS
Passing–Bablok and Deming linear regression analyses
Accuracy. Accuracy was calculated from National Gly-
and Bland–Altman bias testing were performed in
cohemoglobin Standardization Program (NGSP)
Analyse-it Standard Edition for Microsoft Excel
quarterly monitoring and annual accreditation testing.
(Analyse-it Software, Kruskal–
Annual accreditation accuracy testing used 40 unique
Wallis, Dunns, and further Bland–Altman testing were
samples, 8 analyzed in duplicate per day (testing
performed by using GraphPad Prism 5 (GraphPad
spanned 5 days). For quarterly accuracy monitoring,
Software, Statistical significance
10 samples were tested once daily for 2 days. Our accu-
was defined as
P ⬍ 0.05.
racy was assessed as the fractional error [(%Hb A1cFP ⫺ %Hb A1c NGSP)/%Hb A1c NGSP] between the
SAMPLE COLLECTION FOR EPIDEMIOLOGIC STUDIES
mean we obtained for a sample and that measured by
Approximately 700 collection centers in 78 countries
the NGSP reference laboratory.
followed standardized sample collection and han-
Readable area range. A WB sample with an Hb A
dling protocols provided by the CRCTL for both the
6.1% was diluted 1 in 2 with diluent. Seven serial dilu-
INTERHEART (a study of the effect of potentially
tions of this sample in hemolyzing buffer (1 in 4 to
modifiable risk factors associated with myocardial in-
approximately 1 in 20) were used to determine the
farction in 52 countries)
(21 ) and Clopidogrel in Un-
range of readable area that produced a reliable Hb A
stable Angina to Prevent Recurrent Events (CURE)
result (see Fig. 1 in the Data Supplement that accom-
(22 ) studies. Research ethics review boards at each lo-
panies the online version of this article at
cal site approved the study protocols, and all partici-
pants provided informed consent before specimen col-
were blotted on FP, eluted, and analyzed to determine
lection. When venipuncture was performed for
the following: Hb A
collection of clinical specimens, an additional tube of
1c peak area, percent Hb A1c, and
the acceptability of the chromatograph and reported
WB was collected in an EDTA Vacutainer Tube (Bec-
ton Dickinson, and mixed by inver-
sion, and approximately 50 L (1 drop) was applied to
Linearity. Linearity was evaluated by using 22 WB sam-
FP collection cards (Roche, Each FP
ples, each prepared as 10 dilutions with homologous
was allowed to air dry for 2 h, sealed in an individual
plasma (see Table 1 in the online Data Supplement) to
resealable plastic bag, and frozen locally at ⫺20 °C for
determine whether %Hb A
1c is affected by total hemo-
3 months (based on in-house stability data) or
globin concentration. Each dilution of each sample was
⫺70 °C for ⱕ6 months. A total of 15 855 FPs were
blotted onto FP, air-dried, frozen overnight at ⫺70 °C,
shipped frozen on ice packs to the CRCTL and stored at
thawed, and analyzed.
⫺70 °C until analysis.
WB VS FP METHOD COMPARISON
MORPHOMETRIC ANALYSIS OF BORDERLINE CHROMATOGRAPHS
Eighty-five routine clinical WB specimens were se-
In an attempt to improve objectivity in the evaluation
lected on the basis of an initial Hb A1c result, with em-
of peak integration, 100 chromatographs of varying
phasis on the clinically relevant Hb A1c range (approx-
quality were reviewed 3 times by 5 operators (3 experi-
imately 5.5% to 8.5%), stored at 4 °C, blotted on FP,
enced, 2 naive). Chromatographs were classified on the
and analyzed within 96 h of sample collection. Each FP
basis of acceptability of Hb A1c peak integration: those
Clinical Chemistry 57:4 (2011)
defined by ⱖ4 operators as accepted or rejected wereclassified accordingly, and images were deemed bor-
Table 2. Bio-Rad Variant II 6.5-min
derline if multiple operators did not consistently ac-
(
-thalassemia method) HbA /HbA Dual
cept/reject a chromatograph when shown it 3 times in a
Program interassay validation data.a
blinded trial. This resulted in a subset of 25 "border-line" chromatographs. Morphometric analysis of this
subset (Adobe Photoshop 7, deter-
mined the area of the integration peak, nonintegrated
Bio-Rad control 1
area (between the integration peak and the elution
Bio-Rad control 2
peak), and the total area under the elution peak. Area
measurements were restricted to the region within the
Hb A1c retention time window (defined by the instru-
ment as 0.83 ⬍
t ⬍ 1.03 min on the
x axis). Maximum
integration peak height and total width of the bell-
shaped curve (trough-to-trough) were also measured.
Subsequent analysis revealed that the 25 chromato-
graphs consistently possessed poorly integrated Hb
1c peaks. From these, 14 borderline chromato-
graphs displaying only the "poor integration" trait
(without other confounding traits described in Ta-
ble 1) were selected to further quantify this subjec-
tive feature. To estimate interchromatograph error
associated with the morphometric analysis, area
measurements were repeated 10 times on a singlechromatograph and CV% was calculated for inte-
grated, nonintegrated, and total areas.
a Mean, SD, and CV% calculated across all 3 Variant II instruments.
BIO-RAD VARIANT II/6.5-MIN PROGRAM VALIDATION
matograph elution patterns when total area of analysis
In-house prepared FP samples at both normal (ap-
was between 0.8 and 5.5 ⫻ 106 V/s (compared to Bio-
proximately 5.6%) and high (approximately 8.0%) Hb
Rad's suggested range of 1.5–3.5 ⫻ 106 V/s).
Linearity testing of FP blotted with WB samples
1c values yielded excellent intraassay (CV% ⱕ1.84%
and 1.29%, respectively) and interassay (CV% ⱕ1.60%
prediluted with homologous plasma yielded Deming
and 1.23%, respectively) precision. Interassay preci-
regression equations with a mean slope of ⫺3.83 ⫻
sion testing across all QC samples (on all instruments)
10⫺3 (range ⫺5.7 ⫻ 10⫺2 to 7.0 ⫻ 10⫺2). The mean
generated CV% of ⱕ2.00%. Table 2 provides a sum-
fractional error [(%Hb A1c of diluted sample ⫺ %Hb
mary indicating that all data from all 3 instruments are
A1c of neat sample)/%Hb A1c of neat sample] between
consistent with excellent performance. The instru-
each dilution and its neat sample was ⫺0.0026 (mean
ments performed well, meeting the intralaboratory im-
absolute fractional error of 0.0108) with a maximum of
precision specifications recommended by Sacks et al.
0.0488, indicating that sample values were virtually un-
(23 ) and Bio-Rad (⬍3% and ⱕ4%, respectively).
affected by dilutions as great as 2 in 5.
Accuracy assessment from NGSP accreditation
monitoring across the Hb A1c range of 4.45% to 13.5%
WB VS FP METHOD COMPARISON
revealed an increasing negative bias (range of 0.02% to
All chromatographs for WB and matching FP were ac-
⫺0.73%; fractional error range of 0.0034 to ⫺0.0602;
ceptable according to the criteria in Table 1. A Passing–
see Table 2 in the online Data Supplement) with
Bablok agreement plot and Bland–Altman method
DCCT-referenced Hb A
comparison for 85 WB samples and matching FP sam-
1c values. When focused on the
clinically significant range (Hb A ⱕ
ples revealed little difference between the sample types
negative bias was ⫺0.1% (fractional error ⫽ ⫺0.0128).
[(FP Hb A1c) ⫽ 0.933(WB Hb A1c) ⫹ 0.4] (Fig. 1A)
This level of accuracy meets the level I standard for
with a slight negative bias [percent difference ⫽
NGSP accreditation [accuracy, lower 95%, upper 95%
⫺1.66% (1.94%)] (Fig. 1B). However, when the com-
parison of FP to WB was restricted to the 51 samples in
Readable area range experimentation demon-
the clinically significant range (ⱕ8.5%), linear regres-
strated consistently acceptable Hb A1c results and chro-
sion indicated less negative bias [(FP Hb A1c) ⫽
Clinical Chemistry 57:4 (2011)
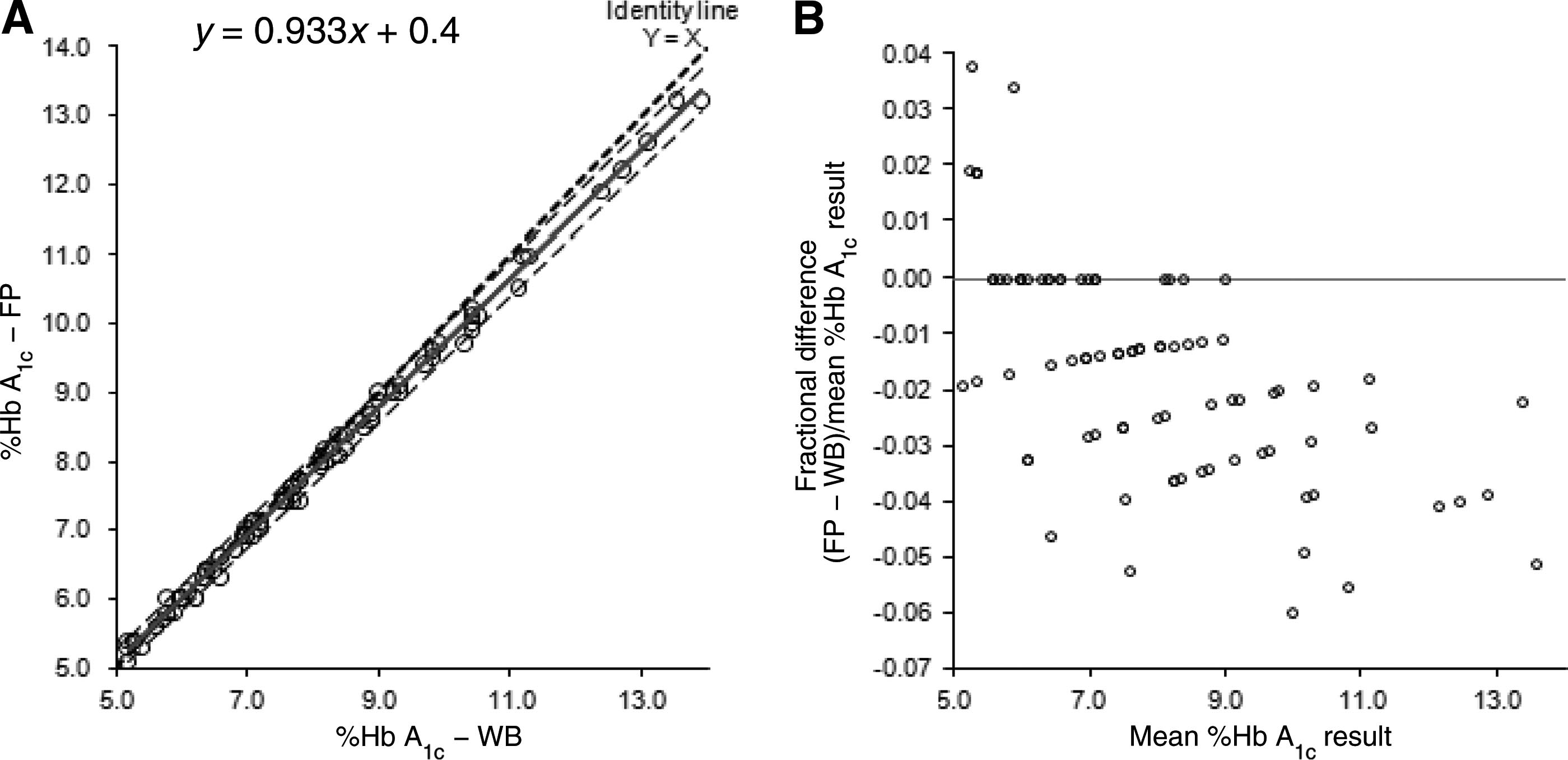
Measurement from Filter Papers in Population Studies
Fig. 1. Passing–Bablok and Bland–Altman analyses of WB versus FP results.
Passing–Bablok method comparison (A) and Bland–Altman bias plot (B) of FP samples (n ⫽ 85) indicated a slight negative biasrelative to WB. The solid line represents the Passing–Bablok trend line (A) or Bland–Altman identity line (B). Unlabelled dashedlines are 95% CIs (A).
0.941(WB Hb A1c) ⫹ 0.353; bias, percent difference ⫽
peak (Fig. 3). Accepted images had significantly lower
⫺0.83 (1.8)%].
(P ⬍ 0.01) percentages of nonintegrated Hb A1c peakarea [20.54% (12.17%)] than high mean percent non-
FP STABILITY
integrated areas [44.16% (7.28%)] for those classified
In-house FP QC samples stored at ⫺70 °C showed vir-
as rejected (Fig. 3). The mean percent nonintegrated
tually no degradation over 3 years (CV% 1.72–2.73)
area for the "borderline" subset of chromatographs was
(see Table 3 in the online Data Supplement). Deming
intermediate [35.40% (3.28%)] to and significantly
linear regression analysis for each control yielded
different (P ⬍ 0.05) from the accepted and rejected
slopes with a range of ⫺1.08 ⫻ 10⫺4 to 7.81 ⫻ 10⫺4
groups. The variation for 10 measurements of total in-
(Fig. 2). Chromatographs from these samples had sim-
tegrated, nonintegrated, and total area on a single chro-
ilar elution patterns and consistent total area counts.
matograph was minimal (CVs ⱕ1.68%), indicating theautomated area-counting tool provided reproducible
LARGE-SCALE STUDY APPLICABILITY
area (pixel) counts and is a valid means for data acqui-
To validate the applicability of the decision rules (Table
sition and assessment of integration (see Table 4 in the
1), we reviewed our Hb A1c data from 2 major multi-
online Data Supplement).
national studies, INTERHEART (21 ) (n ⫽ 11 127) and
The criterion "inadequate separation" could not
CURE (22 ) (n ⫽ 4728). This review was undertaken to
be quantified by using the morphometric tool. The
assess the rate of FP sample repeat (following a single
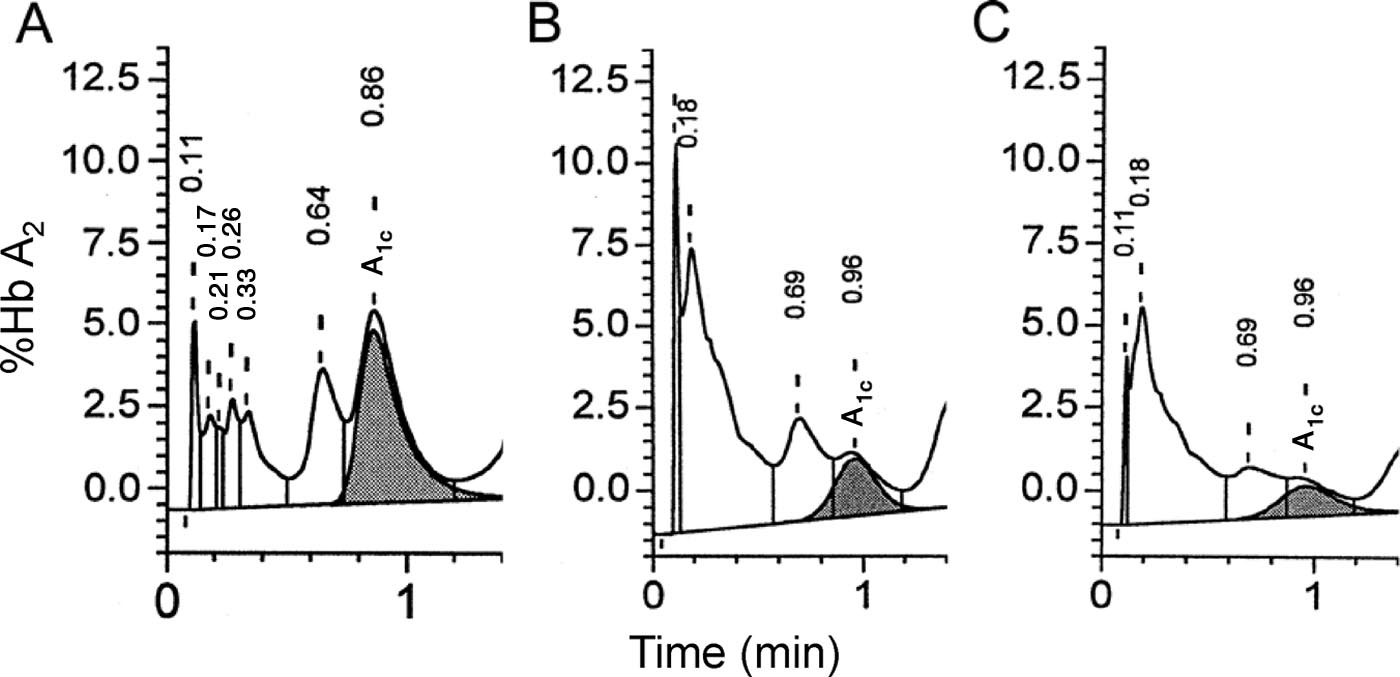
spectrum for the degree of separation criteria is illus-
test) as well as the number of nonreportable samples.
trated in Fig. 4 by chromatographs of 3 different spec-
Evaluation of the chromatographs from these studies
imens. The 3 chromatographs exhibit distinct differ-
revealed an initial repeat rate (based on a single test) of
ences in the degree of labile Hb A1c (LA1c) and Hb A1c
16.57% and 13.64%, respectively, indicating approxi-
separation, such that the result illustrated in Fig. 4A is
mately 85% of samples collected under field conditions
acceptable, the result in 4B would be repeated/reevalu-
were reported with confidence on a single test. After
ated, and the result in 4C would be rejected according
repeat testing, 84.7% of INTERHEART and 96.8% of
to the criteria listed in Table 1.
CURE specimens were reported with confidence.
MORPHOMETRIC ANALYSIS OF BORDERLINE CHROMATOGRAPHS
Morphometric analysis of 42 chromatographs (14 ac-
Numerous methods exist for the determination of WB
cepted, 14 borderline, and 14 rejected) revealed strik-
Hb A1c, including column chromatography, electro-
ing differences between these 3 groups in Hb A1c peak
phoresis and isoelectric focusing, and colorimetric and
integration relative to the total area beneath the elution
immunoassays (24 –26 ). Ion-exchange HPLC methods
Clinical Chemistry 57:4 (2011)

Fig. 2. Long-term stability data for in-house prepared FP quality controls.
Both normal FP controls (A and C) and high FP controls (B and D) were stable at ⫺70 °C for up to 3.25 years. Solid center linesrepresenting Deming regression lines are flanked by curves indicating proportional variance. Outermost lines are 95% CIs.
allow Hb A1c determination without interference from
ble results to those acquired using WB. Our study ex-
its Schiff base (LA1c) and can be used for variant
tends these findings and validates the use of FP samples
screening (27, 28 ). Automated HPLC instruments al-
collected under field conditions in 78 countries from
low rapid and reproducible analysis of samples, appro-
patients enrolled in large, multinational, population-
priate for large population-based studies.
based studies. The Bio-Rad Variant II instrument, us-
Previous evaluation of the Bio-Rad Variant II
ing the 6.5-min (-thalassemia) Variant II HbA2/
yielded intra- and interassay precision of ⬍5% (28 )
HbA1c Dual Program, is a superior method for Hb A1c
and demonstrated the utility and validity of the dual
measurement in a central laboratory for FP blotted
program for measurement of Hb A1c from routine clin-
ical WB samples (29, 30 ). We identified 2 limitations
Our data quantify the Bio-Rad Variant II 6.5-
affecting our large-scale epidemiologic studies using
min (-thalassemia) HbA2/HbA1c Dual Program ac-
FP samples: a high repeat rate for samples collected
curacy, precision, and robustness. Intraassay preci-
under variable conditions and a negative bias in the
sion was consistently ⬍2%, and ⬎90% of interassay
high end (ⱖ8.5 %Hb A1c) of the DCCT range for FP
CV% values were ⬍2%. Dilution experiments indi-
samples relative to WB.
cated consistent and reproducible %Hb A1c results
Earlier (17 ) and more recent (31 ) reports indicate
across a broad range of sample dilutions, and the
that Hb A1c analysis using FP samples yields compara-
reportable total area range (V/s) on the instrument
Clinical Chemistry 57:4 (2011)
Measurement from Filter Papers in Population Studies
on the Bio-Rad Variant II indicated a slight negative
1c results from FP samples in compari-
son to their NGSP reference value (⫺0.1%; mean frac-
tional error of ⫺0.0128 within the clinically significant
range). The CRCTL has held level I NGSP accreditation
on both WB and FP Hb A
1c samples for the past 5 years,
confirming the long-term accuracy and precision of
this method.
1c samples frozen at ⫺70 °C have been
shown to provide reliable results after a decade of stor-
age (15 ). Earlier work indicated FP samples remainstable at ⫺70 °C for several months (17 ). Our data in-
Fig. 3. Quantification of nonintegrated area of ac-
dicate that FP blotted with WB and stored at ⫺70 °C
cepted, borderline, and rejected chromatographs.
maintained sample integrity and yielded CVs ⱕ2.73%
Mean percent nonintegrated areas [(nonintegrated area/
over approximately 3 years, supporting research facili-
total elution area) ⫻ 100%] for each group (n ⫽ 14 each)
ties and/or biorepositories storing samples at ⫺70 °C
of chromatographs (accepted, borderline, and rejected)
over many years.
were found to be significantly different from one another
Large-scale/multinational population-based stud-
based on a Kruskal–Wallis ranked ANOVA and Dunn's
ies present difficulties not normally encountered dur-
post-hoc analysis (*P ⬍ 0.05, **P ⬍ 0.01).
ing routine clinical analysis. Although standardizedprotocols are provided to sample collection sites, sam-ples collected in global studies may be subjected to po-
accommodated both dilute and concentrated FP el-
tentially degenerative effects of harsh preanalytical
uates, as reported by Higgins et al. (28 ). This is im-
conditions. Nevertheless, the Bio-Rad Variant II gen-
portant because our experience with FP collection
erated reportable values for approximately 83% and
on a global scale indicates some WB samples settle
approximately 86% for INTERHEART and CURE,
before blotting on FP, resulting in a concentrated
respectively, of 15 855 FP samples on the first test.
sample drawn from the erythrocytes in the bottom of
INTERHEART samples were frequently rejected for is-
the tube, or a dilute sample drawn near the top of the
sues related to the quality of sample preparation and
preanalytical sample degradation (i.e., Hb A1c shoul-
NGSP accreditation monitoring of the 6.5-min
dering, high LA1c, very low area counts), whereas
(-thalassemia) Variant II HbA2/HbA1c Dual Program
CURE specimens were rejected for instrument pro-
Fig. 4. Hb A
elution peak separation.
(A), "Accepted": well separated with a distinct trough between the LA
(peak to immediate left of Hb A
crest. (B), "Borderline" on the basis of "inadequate separation" (Table 1). (C), "Rejected" chromatograph based oninadequately separated LA
crest not distinct.
Clinical Chemistry 57:4 (2011)
cessing errors (i.e., software exceptions). This result
In summary, the data presented in this report
is exemplified by the disparity in final rates of accep-
validate both the collection of WB on FP for Hb A1c
tance (approximately 85% and approximately 97% for
determination in large-scale population studies and
INTERHEART and CURE, respectively), because most
testing of these FP samples on the Bio-Rad Variant II
first-pass rejections in the CURE study were resolved
using the 6.5-min (-thalassemia) Variant II HbA2/
on repeat. Improper local preparation (e.g., inadequate
HbA1c Dual Program in a level I NGSP-accredited lab-
postblotting dry-time and high humidity during pre-
oratory. The negative bias in FP results compared to
transport packaging) of INTERHEART samples re-
WB is negligible and does not affect clinical decisions
sulted in reduced specimen quality relative to CURE
(Fig. 1). The utility of FP collection under field condi-
specimens. Furthermore, INTERHEART samples were
tions makes worldwide sample collection for Hb A1c
collected in smaller and more remote locations com-
testing feasible. However, it is imperative that person-
pared to the larger collection centers in CURE. Al-
nel in the field receive adequate training and under-
though after repeat, up to approximately 97% of sam-
stand the importance of consistent collection, han-
ples of the CURE study were reported with confidence,
dling, freezer storage, and shipment practices, to avoid
the initial repeat rates are not ideal. To repeat approx-
high nonreportable rates at analysis. In addition, we
imately 11% and reject ⬎10% of a large study popula-
anticipate that our approach to the quantification and
tion is costly; all efforts must be made to ensure proper
visual representation of qualitative and highly subjec-
sample preparation and preservation. Central analysis
tive chromatograph traits deemed "reasons for repeat/
of Hb A1c from properly prepared FP collected in pop-
rejection" will reduce interoperator decision-making
ulation studies is cost-effective and eliminates variabil-
variability and improve the analysis and reporting of
ity due to use of different analytical methods at multi-
Hb A1c values.
ple laboratories.
The performance of the 6.5-min (-thalassemia
method) Variant II HbA2/HbA1c Dual Program on theBio-Rad Variant II exceeded the 1.5-min (Turbo) and
Author Contributions: All authors confirmed they have contributed to
the intellectual content of this paper and have met the following 3 re-
3-min Hb A1c programs on the Variant II, which did
quirements: (a) significant contributions to the conception and design,
not sufficiently separate the hemoglobins in eluted FP
acquisition of data, or analysis and interpretation of data; (b) drafting
samples, resulting in abnormal elution patterns, poorly
or revising the article for intellectual content; and (c) final approval of
integrated peaks, identification of false variants, and
the published article.
erroneous Hb A1c results (internal data; see Fig. 2 in the
Authors' Disclosures or Potential Conflicts of Interest: Upon man-
online Data Supplement).
uscript submission, all authors completed the Disclosures of Potential
Blinded interoperator chromatograph analysis
Conflict of Interest form. Potential conflicts of interest:
showed that ambiguous criteria were those that lacked
Employment or Leadership: J.L. Keys, Clinical Research and Clini-
numerical definitions (Table 1). To refine these subjec-
cal Trials Laboratory and Hamilton Health Sciences; S.K. Hall, Clin-
tive repeat/rejection criteria, morphometric analysis of
ical Research and Clinical Trials Laboratory and Hamilton Health
accepted, rejected, and borderline chromatographs re-
Sciences.
Consultant or Advisory Role: None declared.
vealed significant differences (P ⬍ 0.05) in the percent
Stock Ownership: None declared.
nonintegrated area (nonintegrated area/total area) be-
Honoraria: None declared.
tween the 3 groups of chromatographs. Those classified
Research Funding: None declared.
as accepted were consistently better integrated (mean
Expert Testimony: None declared.
20.54% nonintegrated) than the borderline (35.40%)
Role of Sponsor: The funding organizations played no role in the
or rejected (44.16%) chromatographs. The error asso-
design of study, choice of enrolled patients, review and interpretation
ciated with the morphometric analysis yielded CVs of
of data, or preparation or approval of manuscript.
ⱕ1.68% for area measurements. It was not possible to
Acknowledgments: The authors thank the staff of the Clinical Re-
quantify inadequate separation, the other qualitative
search and Clinical Trials Laboratory for technical contributions and
rejection criterion, although a clear visual distinction
support. We also specifically acknowledge Linda Carr who collectedpreliminary validation data and analyzed specimens for both the
between accepted, borderline, and rejected elution pat-
INTERHEART and CURE studies, Karen Bamford who assisted in
terns is present (Fig. 4 and Fig. 2 in the online Data
data collection, and the Special Chemistry Department at the Ham-
ilton General Hospital's Core Laboratory.
1. International Diabetes Federation. IDF diabetes
diabetes, 1995–2025: prevalence, numerical esti-
Group. The effect of intensive treatment of diabetes
atlas. 4th ed. Brussels (Belgium): International
mates, and projections. Diabetes Care 1998;21:
on the development and progression of diabetes on
Diabetes Federation; 2009.
the development and progression of long term com-
2. King H, Aubert RE, Herman WH. Global burden of
3. Diabetes Control and Complications Trial Research
plications in insulin-dependent diabetes mellitus.
Clinical Chemistry 57:4 (2011)
Measurement from Filter Papers in Population Studies
N Engl J Med 1993;329:977– 86.
stepwise plasma glucose change over time in
22. Keltai M, Tonelli M, Mann JF, Sitkei E, Lewis BS,
4. Diabetes Control and Complications Trial/Epide-
Hawken S, et al. Renal function and outcomes in
miology of Diabetes Interventions and Complica-
acute coronary syndrome: impact of clopidogrel.
tions Research Group. Retinopathy and nephrop-
14. Little R, Rohlfing C, Tennill A, Connolly S, Hanson
Eur J Cardiovasc Prev Rehabil 2007;14:312– 8.
athy in patients with type 1 diabetes four years
S. Effects of sample storage conditions on gly-
23. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK,
after a trial of intensive therapy. N Engl J Med
cated hemoglobin measurement: evaluation of
McDonald JM, Parrott M. Guidelines and recom-
five different high performance liquid chromatog-
mendations for laboratory analysis in the diagno-
5. Cleary PA, Orchard TJ, Genuth S, Wong ND, De-
raphy methods. Diabetes Technol Ther 2007;9:
sis and management of diabetes mellitus. Clin
trano R, Backlund JC, et al. The effect of intensive
Chem 2002;48:436 –72.
glycemic treatment on coronary artery calcifica-
15. Selvin E, Coresh J, Jordahl J, Boland L, Steffes
24. Goldstein D, Little R, Lorenz R, Malone J, Nathan
tion in type 1 diabetic participants of the Diabetes
MW. Stability of haemoglobin A1c (HbA1c) mea-
D, Peterson C, et al. Tests of glycemia in diabetes.
Control and Complications Trial/Epidemiology of
surements from frozen whole blood samples
Diabetes Care 2004;27:1761–73.
Diabetes Interventions and Complications (DCCT/
stored for over a decade. Diabet Med 2005;22:
25. Lahousen T, Roller RE, Lipp RW, Schnedl WJ.
EDIC) study. Diabetes 2006;55:3556 – 65.
Silent haemoglobin variants and determination of
6. Krishnamurti U, Steffes MW. Glycohemoglobin: a
16. Wikblad K, Smide B, Bergstrom A, Wahren L,
HbA1c with the HPLC Bio-Rad Variant II. J Clin
primary predictor of the development or reversal
Mugusi F, Jeppsson JO. Immediate assessment of
Pathol 2002;55:699 –703.
of complications of diabetes mellitus. Clin Chem
HbA1c under field conditions in Tanzania. Diabe-
26. Joutovsky A, Hadzi-Nesic J, Nardi M. HPLC reten-
2001;47:1157– 65.
tes Res Clin Pract 1998;40:123– 8.
tion time as a diagnostic tool for hemoglobin
7. Rohlfing CL, Little RR, Wiedmeyer HM, England
17. Jeppsson JO, Jerntorp P, Almer LO, Persson R,
variants and hemoglobinopathies: a study of
JD, Madsen R, Harris MI, et al. Use of GHb
Ekberg G, Sundkvist G. Capillary blood on filter
60,000 samples in a clinical diagnostic labora-
(HbA1c) in screening for undiagnosed diabetes in
paper for determination of HbA1c by ion ex-
tory. Clin Chem 2004;50:1736 – 47.
the U.S. population. Diabetes Care 2000;23:187–
change chromatography. Diabetes Care 1996;19:
27. Higgins T, Ridley B. Tentative identification of
hemoglobin variants in the Bio-Rad Variant II
8. Chandalia HB, Krishnaswamy PR. Glycated hemo-
18. Anjali, Geethanjali FS, Kumar RS, Seshadri MS.
HbA1c method. Clin Biochem 2005;38:272–7.
globin. Curr Sci 2002;83:1522–32.
Accuracy of filter paper method for measuring
28. Higgins TN, Blakney GB, Dayton J. Analytical
9. Eckfeldt JH, Bruns DE. Another step toward stan-
glycated hemoglobin. J Assoc Physicians India
evaluation of the Bio-Rad Variant II automated
dardization of methods for measuring hemoglo-
HbA1C analyzer. Clin Biochem 2001;34:361–5.
bin A1c. Clin Chem 1997;43:1811–3.
19. Parkes J, Ray R, Kerestan S, Davis H, Ginsberg B.
29. Lafferty JD, McFarlane AG, Chui DHK. Evaluation
10. Nathan DM, Singer DE, Hurxthal K, Goodson JD.
Prospective evaluation of accuracy, precision, and
of a dual hemoglobin A2/A1c quantitation kit on
The clinical information value of the glycosylated
reproducibility of an at-home hemoglobin A1c
the Bio-Rad Variant II automated hemoglobin
hemoglobin assay. N Engl J Med 1984;310:
sampling kit. Diabetes Technol Ther 1999;1:
analyzer. Arch Pathol Lab Med 2002;126:1494 –
11. Nathan DM, Turgeon H, Regan S. Relationship
20. Fokkema MR, Bakker AJ, de Boer F, Kooistra J, de
30. Moridani M, Verjee Z, Allen L. Analytical evalu-
between glycated haemoglobin levels and mean
Vries S, Wolthuis A. HbA1c measurements from
ation of hemoglobin A1c dual kit assay on Bio-
glucose levels over time. Diabetologia 2007;50:
dried blood spots: validation and patient satisfac-
Rad Variant II: an automated HPLC hemoglobin
2239 – 44.
tion. Clin Chem Lab Med 2009;47:1259 – 64.
analyzer for the management of diabetic pa-
12. Fitzgibbons JF, Kolar RD, Jones RT. Red cell age-
21. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A,
tients. Clin Biochem 2003;36:317–20.
related changes of hemoglobins A1a⫹b and A1c
Lanas F, et al. Effect of potentially modifiable risk
31. Jones TG, Warber KD, Roberts BD. Analysis of
in normal and diabetic patients. J Clin Invest
factors associated with myocardial infarction in
hemoglobin A1c from dried blood spot samples
1976;58:820 – 4.
52 countries (the INTERHEART study): case-
with the Tina-quant II immunoturbidimetric
13. Tahara Y, Shima K. The response of GHb to
control study. Lancet 2004;364:937–52.
method. J Diabetes Sci Technol 2010;4:244 –9.
Clinical Chemistry 57:4 (2011)
Source: http://www.viapost.nu/ovrigt/artikel.pdf
December 8, 2008 We have polled our analysts globally to identify the highest quality companies in their sectors, given that an increasing number of stocks appear to be dislocated from their fundamental valuations. Our driving principle was to create a list of 50 companies whose business models and market positions one would like to have had
Estudios Constitucionales, Año 8, Nº 2, 2010, pp. 633 - 674. Centro de Estudios Constitucionales de Chile Universidad de Talca "Informe en derecho presentado ante el Tribunal Constitucional en el proceso de inconstitucionalidad del artículo 38 ter de la Ley N° 18.933" Pablo Contreras V., Gonzalo García P., Tomás Jordán D., Álvaro Villanueva R. INFoRME EN DERECHo PRESENTADo







