
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Hallmarks of cancer and implications to therapeutic applicability
Josep Tabernero, MD, PhD
Moderator
Case Forum
mCRC – Case Study 1
Fortunato Ciardiello, MD, PhD
Second University of Naples
Naples, Italy
Case Study 1
Fortunato Ciardiello, MD, PhD


Meet Sandra
Sandra is a 69-year-old woman with rectal cancer.
At the time of diagnosis in early 2010, her only medical
condition was hypertension.
Although she was initially treated with total resection and
adjuvant chemotherapy, she experienced liver recurrence in
2011.
Treatment of her metastatic disease was multidisciplinary
and included metastectomy and 3 lines of systemic therapy.
Interestingly, her third-line treatment offered longer PFS than
her first-line treatment.
Case courtesy of Dr Fortunato Ciardiello.
Sandra: 69-Year-Old Woman with RAS-MT mRC
Comorbidities: Hypertension in treatment with amlodipine 5 mg and nebivolol 5 mg.
February 2010: Initial diagnosis and excision of mucinous adenocarcinoma pT4 N2 (7/18) M0KRAS-mutated G12V
July 2011: CT scan showed PD – new liver mets (VII and VIII segment)
Adjuvant chemotherapy FOLFOX (12 cycles)
Case courtesy of Dr Fortunato Ciardiello.
Sandra: 69-Year-Old Woman with RAS-MT mRC
Comorbidities: Hypertension in treatment with amlodipine 5 mg and nebivolol 5 mg.
February 2010: Initial diagnosis and excision of mucinous adenocarcinoma pT4 N2 (7/18) M0KRAS-mutated G12V
July 2011: CT scan showed PD – new liver mets (VII and VIII segment)
Adjuvant chemotherapy
FOLFIRI + Bevacizumab
FOLFOX (12 cycles)
Case courtesy of Dr Fortunato Ciardiello.
Sandra: 69-Year-Old Woman with RAS-MT mRC
Comorbidities: Hypertension in treatment with amlodipine 5 mg and nebivolol 5 mg.
February 2010: Initial diagnosis and excision of mucinous adenocarcinoma pT4 N2 (7/18) M0 KRAS-mutated G12V
February 2013: CT scan showed diaphragmatic and nodal PD
July 2011: CT scan showed PD – new liver mets (VII and
October 2012: CT scan
showed lymph node PD
Adjuvant chemotherapy
FOLFIRI + Bevacizumab
FOLFOX (12 cycles)
Case courtesy of Dr Fortunato Ciardiello.
Assuming regorafenib is approved and reimbursed in your country, which therapy are you likely to use next on this patient if her performance status is good?
1. FOLFOXIRI (± bevacizumab)
3. (FOLF)IRI + bevacizumab or ziv-aflibercept
Case courtesy of Dr Fortunato Ciardiello.
Why Regorafenib for Sandra in the Third-Line?
• Need for an effective third-line therapy
• She is in good performance status and is willing to
undergo treatment
Case courtesy of Dr Fortunato Ciardiello.
First dose of regorafenib on March 5, 2013
160 mg/day, 3 weeks on/1 week off
Case courtesy of Dr Fortunato Ciardiello.
In third-line or later treatment of mCRC, in general, which of the following is the most realistic therapeutic goal in terms of tumor response?
1. Complete response
2. Partial response
3. Stable disease
4. Retained or improved QOL, regardless of tumor response
Case courtesy of Dr Fortunato Ciardiello.
Sandra – Tumor Assessment
Multiple lymph nodes
Diaphragmatic lesion 18 × 27 mm
(perihepatic, paraortic)
Case courtesy of Dr Fortunato Ciardiello.
Sandra – Main Toxicities
Diarrhea G1; Hypophosphatemia G2
Hand-foot syndrome G1
Case courtesy of Dr Fortunato Ciardiello.
Topical Treatment Considerations for HFSR
Frequently apply emollients and creams to maintain skin moisture,
prevent cracks/breaks, and provide relief1-3• Consider adding local topical steroids, such as clobetasol 0.05%
ointment, to erythematous areas twice daily1,2
• Topical analgesics to manage pain1,2 • More intense topical therapies for symptomatic relief1-3
• For severe HFSR, a combination of cortisone cream and topical
1. Lacouture ME, et al. Oncologist. 2008;13:1001-1011; 2. Wood LS, et al. Commun Oncol. 2010;7:23-29; 3. Anderson R, et al. Oncologist. 2009;14:291-302.
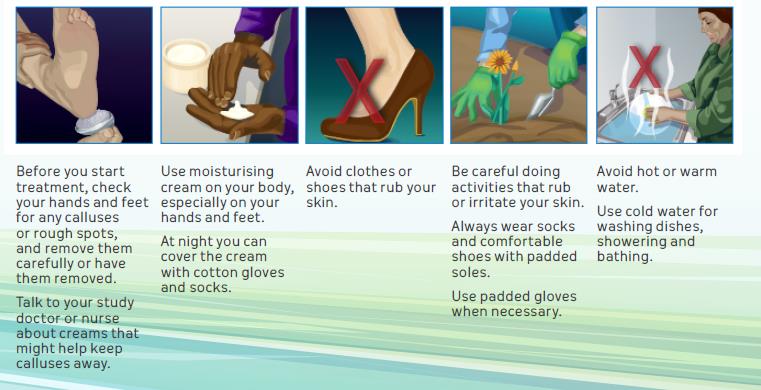
Patient Guidance on HFSR: Steps that May Help
Manage Symptoms
Lacouture ME, et al. Oncologist. 2008;13:1001-1011; Khan G, et al. Cancer Manage Res. 2014;6:93-103.
Sandra – Main Toxicities
Diarrhea G1; Hypophosphatemia G2
Hand-foot syndrome G1
Hand-foot syndrome G2
Hand-foot syndrome G3
Case courtesy of Dr Fortunato Ciardiello.
Recommended Dose Modifications and Measures for
HFSR
Skin Toxicity Occurrence
Recommended Dose Modification and Measures
• Institute supportive measures immediately. Interrupt therapy for a
minimum of 7 days until toxicity resolves to Grade 0–1
• When re-starting treatment, decrease dose by 40 mg (one tablet)• A dose re-escalation is permitted at the discretion of the physician
• Institute supportive measures immediately. Interrupt therapy for a
minimum of 7 days until toxicity resolves to Grade 0–1
• When re-starting treatment, decrease dose by 40 mg (one tablet)
• Discontinue treatment with regorafenib permanently
Stivarga (regorafenib) EMA SmPC, January 2015.
Sandra – Main Toxicities
Diarrhea G1; Hypophosphatemia G2
Hand-foot syndrome G1
Hand-foot syndrome G2
Hand-foot syndrome G3
1 dose reduction level
Hand-foot syndrome G1
Hand-foot syndrome G1
Hypophosphatemia G2
Case courtesy of Dr Fortunato Ciardiello.
Sandra: 69-Year-Old Woman with RAS-MT mRC
Comorbidities: Hypertension in treatment with amlodipine 5 mg and nebivolol 5 mg.
February 2010: Initial diagnosis and excision of mucinous adenocarcinoma pT4 N2 (7/18) M0 KRAS-mutated G12V
February 2013: CT scan showed diaphragmatic and nodal PD
July 2011: CT scan showed
July 2014: CT scan showed
PD – new liver mets (VII and
October 2012: CT scan
massive perihepatic and paraortic
showed lymph node PD
lymph nodal progression
Adjuvant chemotherapy
FOLFIRI + Bevacizumab
FOLFOX (12 cycles)
Case courtesy of Dr Fortunato Ciardiello.
In July 2014, After 17 Cycles of Treatment
• Sandra was discontinued for clinical progression
– Abdominal pain G3
– Hyperbilirubinemia G3
• Confirmed by abdominal CT scan
– Massive perihepatic and paraortic lymph nodal progression
• Sandra died on October 24, 2014
– OS from initiation of third-line therapy: 19 months
Case courtesy of Dr Fortunato Ciardiello.
Key Takeaways from Sandra's Experience
• Important not to wait – Sandra received regorafenib as soon as she
was eligible for treatment
• She was a good candidate for regorafenib because she was in good
performance status (0-1)
• Implemented a plan to monitor her frequently in order to evaluate,
prevent, and manage AEs, to keep her on therapy
– Aggressively monitor her for the first 2 weeks of regorafenib therapy
• Tailor dosing for improved patient tolerability
Total Cycles 17 and 1 dose-level reduction was
required (120 mg/daily) from Cycle 8
Best response: PR
Patient died on October 24, 2014
Case courtesy of Dr Fortunato Ciardiello.

GI Challenge Question
Which of the following tactics would you consider to manage a patient on regorafenib presenting with Grade 2 fatigue?
1. Inform patients about the potential benefits of exercise on
2. Monitor the patient weekly for the first 2 cycles and at every
visit (every 2 weeks) thereafter
3. Consider regorafenib dose interruption
4. Consider regorafenib dose reduction to 120 mg a day
5. All of the above
"Pick-a-Case" in
mCRC
Josep Tabernero, MD, PhD
Moderator
Considerations for Treatment Selection
• Eligible patients need to fulfill 4 criteria
– ECOG performance status 0 or 1– Absence of poorly controlled hypertension– Absence of severe cardiovascular disease in prior 6 months– Adequate bone marrow, liver, and renal functions
• In addition to these criteria, patients must be monitored during
the first days or weeks of treatment with regorafenib for
– Abnormal increase in the level
of hepatic enzymes
Sastre J, et al. Clin Transl Oncol. 2014;16:942-953.

Meet Kevin
Kevin is a 71-year-old man with RAS MT rectal cancer.
After his initial diagnosis in 2004, he was treated with
concurrent chemoRT and surgery and subsequent
adjuvant XELOX, with a 4-year DFS.
Having experienced 16-months progression-free with
first-line chemotherapy/bevacizumab and subsequently
bevacizumab (plus alternate chemotherapy) beyond
progression…
…He has now had 25 months of exposure to the VEGF
inhibitor and is facing disease progression in both the
lungs and pelvis.
Case courtesy of Dr Josep Tabernero.

Meet Hector
Hector is a 72-year-old man with hypertension and diabetes.
In 2011, he was diagnosed with KRAS wildtype colon cancer
and synchronous unresectable liver metastases.
Following removal of the primary, he experienced only very
brief disease stability (3 months) with an oxaliplatin-based
regimen and was treated with irinotecan-based therapies
until he developed lung metastases.
Despite his comorbidities, can close monitoring and
supportive care enable Hector to receive additional therapy
to control his disease?
Case courtesy of Dr Fortunato Ciardiello.


Which Other Case Would You Like to Hear More About?
Kevin
(71-year-old man with RAS MT rectal cancer)
Hector
(72-year-old man with hypertension and diabetes who was
diagnosed with RAS WT metastatic colon cancer)
GI Challenge Question
Which of the following tactics would you consider to manage a patient on regorafenib presenting with Grade 2 asymptomatic hypertension not controlled with the addition of antihypertensive therapy?
Consider increasing frequency of BP monitoring
Reduce regorafenib dose by 1 dose level for at least 1 full cycle; if controlled, consider dose re-escalation
Interrupt study medication until symptoms resolve and diastolic BP is ≤100 mmHg
Increase or institute additional antihypertensive medication
Meet Kevin
Kevin is a 71-year-old man with RAS MT rectal cancer.
After his initial diagnosis in 2004, he was treated with
concurrent chemoRT and surgery and subsequent
adjuvant XELOX, with a 4-year DFS.
Having experienced 16-months progression-free with
first-line chemotherapy/bevacizumab and subsequently
bevacizumab (plus alternate chemotherapy) beyond
progression…
…He has now had 25 months of exposure to the VEGF
inhibitor and is facing disease progression in both the
lungs and pelvis.
Case courtesy of Dr Josep Tabernero.
Kevin: 71-Year-Old Man in Good Performance Status,
Stage IV Rectal Cancer, KRAS MT
Stage IIIB
Adenocarcinoma of the rectum
(pT3 N2a[3/23] M0); Total
mesorectal excision and
new pelvic disease
DFS: 48 mos
PFS: 16 mos
PFS: 9 mos
Second progression
First progression
Case courtesy of Dr Josep Tabernero.
Based on evidence and your practice, which therapy are you likely to use next on this patient?
2. Experimental agent
3. Mitomycin C + capecitabine
4. Retreatment with 5FU + oxaliplatin
Kevin: 71-Year-Old Man, Stage IV Rectal Cancer, KRAS MT
• Treatment with regorafenib 160 mg/day
Case courtesy of Dr Josep Tabernero.
Kevin: 71-Year-Old Man, Stage IV Rectal Cancer, KRAS MT
• G3 Rash
– Start C1D13 G3
Regorafenib put on hold until C2D1
Case courtesy of Dr Josep Tabernero.
When Treating with Regorafenib, Monitor AEs Weekly to
Reach an Efficacy Assessment at 8 weeks
• Regorafenib treatment-emergent AEs most frequently occur in the first 2
cycles of treatment and stabilize or decrease over time1,2
• During the first 2 cycles of regorafenib, patients should be managed with
weekly monitoring and proactive intervention3
– The convenient oral formulation of regorafenib allows for a tailored approach to
• Regorafenib AEs are mostly manageable and generally reversible2
• The recommended starting dose of 160 mg can be reduced in 40-mg
decrements to 120 mg or 80 mg to manage AEs1,4,5
– Upon stabilization of AEs* the dose can be re-escalated at the physician's
*Grade ≤3.
1. Grothey A, et al. ASCO GI 2013. Abstract 467; 2. De Wit M, et al. Support Care Cancer. 2014; 22(3):837-846; 3. Grothey A, et al. Oncologist.
2014;19(6):669-680; 4. Stivarga (regorafenib) EMA SmPC, January 2015; Li J, et al. Lancet Oncol. 2015;16(6):619-629.
Kevin: 71-Year-Old Man, Stage IV Rectal Cancer, KRAS MT
• G3 Rash
– Start C1D13 G3
Regorafenib put on hold until C2D1
– C2D1 fully recovered
Regorafenib was restarted at DL -1 (120 mg)
– C3D1 no further episodes of rash
Regorafenib continued at 120 mg until PD
Case courtesy of Dr Josep Tabernero.
Incidence of Grade 3 AEs Decreases Over Time
Grothey A, et al. ASCO GI 2013. Abstract #467.
Kevin: 71-Year-Old Man, Stage IV Rectal Cancer, KRAS MT
• Treatment with regorafenib 160 mg/day• From July 20 to November 15, 2010• PFS: 4 months• Best response: SD• Safety profile
Dose reduction to 120 mg
No adverse events since dose reduction
Case courtesy of Dr Josep Tabernero.
CORRECT: Drug-Related Treatment-Emergent AEs
Occurring in ≥10% of Patients
Regorafenib + BSC arm
Placebo + BSC arm
Adverse Event, %
All Grades
Grade 3/4
All Grades
Grade 3/4
Severe liver toxicity events have been reported. Liver function testing (ALT, AST, and bilirubin) should occur prior to treatment with regorafenib, every 2 weeks during the first 2 months of treatment, and then monthly or more frequently, as clinically indicated.2
Drug-related adverse reactions that resulted in treatment discontinuation were reported in 8.2% of regorafenib-treated patients compared to 1.2% of patients who received placebo3
1. Grothey A, et al. Lancet. 2013;381:303-312; 2. Stivarga (regorafenib) EMA SmPC, January 2015; Grothey A, et al. Presented
at ASCO GI, 2012, San Francisco, CA.
Kevin: 71-Year-Old Man, Stage IV Rectal Cancer, KRAS MT
Basal CT Scan
C3D1 CT Scan
C5D1 CT Scan
New liver lesions
Case courtesy of Dr Josep Tabernero.
Effects on Tumor Size (CORRECT) and Rates of PR and SD
in CORRECT and CONCUR
Regorafenib
Best response, %
Complete response (CR)
Partial response (PR)
Stable disease (SD)
Progressive disease (PD)
*DCR, disease control rate.*DCR = PR + SD ≥6 weeks after randomization; P<.000001.
1. Van Cutsem E, et al. ESMO 2012. Abstract LBA18; Li J, et al. Lancet Oncol. 2015;16(6):619-629.
• Effective AE managment was achieved through an early
monitoring strategy for patients
– All AEs occurred in Cycle 1 and were manageable
Patient completed 4 cycles of treatment; a best response of
SD was observed
How frequently do you plan to monitor patients you decide to treat with regorafenib?
2. Once a week for the first 2 cycles, and then every 2 weeks
3. Once a week for the first 2 cycles and then monthly
5. "Closely" during the first 6 weeks of therapy
Meet Hector
Hector is a 72-year-old man with hypertension and diabetes.
In 2011, he was diagnosed with KRAS wildtype colon cancer
and synchronous unresectable liver metastases.
Following removal of the primary, he experienced only very
brief disease stability (3 months) with an oxaliplatin-based
regimen and was treated with irinotecan-based therapies
until he developed lung metastases.
Despite his comorbidities, can close monitoring and
supportive care enable Hector to receive additional therapy
to control his disease?
Case courtesy of Dr Fortunato Ciardiello.
Hector: 72-Year-Old Man with Hypertension and Diabetes,
KRAS Wildtype Colon Cancer
Adenocarcinoma of right
colon (pT4 N2[4/12] M1
[liver]) treated with right
PFS: 3 mo
Case courtesy of Dr Fortunato Ciardiello.
Hector: 72-Year-Old Man with Hypertension and Diabetes,
KRAS Wildtype Colon Cancer
Adenocarcinoma of right
colon (pT4 N2[4/12] M1
[liver]) treated with right
PFS: 3 mo
PFS: 8 mo
Case courtesy of Dr Fortunato Ciardiello.
Hector: 72-Year-Old Man with Hypertension and Diabetes,
KRAS Wildtype Colon Cancer
Adenocarcinoma of right
colon (pT4 N2[4/12] M1
Liver progression and
[liver]) treated with right
new lung lesions
PFS: 3 mo
PFS: 8 mo
PFS: 2 mo
Case courtesy of Dr Fortunato Ciardiello.
Based on evidence and your practice, which therapy are you likely to use next on this patient?
1. Regorafenib, if available
2. Experimental agent (clinical trial)
3. Retreatment with 5FU + irinotecan
4. Other treatment
Hector: 72-Year-Old Man with Hypertension and Diabetes,
KRAS Wildtype Colon Cancer
Adenocarcinoma of right
colon (pT4 N2[4/12] M1
Liver progression and
[liver]) treated with right
new lung lesions
PFS: 3 mo
PFS: 8 mo
PFS: 2 mo
Case courtesy of Dr Fortunato Ciardiello.
Hypertension Management: Overview
Effective management is critical to minimize long-term sequelae of MKI-induced hypertension1-3
• Ensure that pre-existing hypertension is controlled before starting regorafenib4
– Increasing the dose of current antihypertensive medication or adding new medication if clinically indicated
• Blood pressure should be monitored regularly during treatment with regorafenib4
– To identify hypertension early, monitor blood pressure weekly for the first 6 weeks of treatment and then
every cycle, or more frequently as clinically indicated
• The patient is advised to have access to a blood pressure monitor at home to record blood
pressure regularly and contact healthcare team for specified elevations5
• In cases of hypertension that remains severe or persistent despite adequate medical
management, regorafenib should be temporarily interrupted and/or its dose should be reduced at the discretion of the treating physician5
Recommendations for managing MKI-induced hypertension5
Manage according to standard medical practice, including use of antihypertensive medication
Dose interruption and dose reduction of regorafenib may be necessary
1. Wood LS. Commun Oncol. 2006;3:558-562; 2. Bellmunt J, et al. Crit Rev Oncol Hematol. 2011;78:24-32; 3. Schwandt A, et al. Onco Targets Ther.
2009;2:51-61; 4. Stivarga (regorafenib) EMA SmPC, January 2015; 5. Grothey A, et al. Oncologist. 2014;19(6):669-680.
Hector: Main Treatment-Emergent AEs by Cycle
Emergent AE
Treatment
G1 voice alteration
No dose modification
G1 ↑ amylase &
No dose modification
Case courtesy of Dr Fortunato Ciardiello.
When Treating with Regorafenib, Monitor AEs Weekly to
Reach an Efficacy Assessment at 8 weeks
• Regorafenib treatment-emergent AEs occur most frequently in the first 2
cycles of treatment and stabilize or decrease over time1
• Regorafenib AEs are mostly manageable and generally reversible2• The 2 phase III trials of regorafenib (CORRECT and CONCUR) were limited to
patients with ECOG performance status 0–11,3,4
• During the first 2 cycles of regorafenib, patients should be managed with
weekly monitoring and proactive intervention5
– The convenient oral formulation of regorafenib allows for a tailored approach to
• The recommended starting dose of 160 mg can be reduced in 40-mg
decrements to 120 mg or 80 mg to manage AEs4
– Upon stabilization of AEs* the dose can be re-escalated at the physician's
*Grade ≤3.
1. Grothey A, et al. ASCO GI; 2013. Abstract 467; 2. De Wit M, et al. Support Care Cancer. 2014; 22(3):837-846; 3. Stivarga (regorafenib) EMA SmPC,
January 2015; 4. Li J, et al. Lancet Oncol. 2015;16(6):619-629; 5. Grothey A, et al. Oncologist. 2014;19(6):669-680.
Liver Function Abnormalities Have Been Associated
with Regorafenib Treatment
• Liver-related AEs have been observed in patients treated with regorafenib
─ Abnormalities have manifested as increased levels of alanine aminotransferase
(ALT), aspartate aminotransferase (AST) and/or bilirubin
─ In a small proportion of patients, observed abnormalities were severe (Grade 3 to 4)
• Early detection of liver function abnormalities requires active patient
─ Liver function testing (ALT, AST, and bilirubin) should occur prior to treatment
with regorafenib, and also during the first 2 months of treatment
─ Thereafter, periodic monitoring should occur at least monthly, as clinically
• Changes in liver function may require modifications to regorafenib dosing or
an interruption and even in some cases a discontinuation of treatment
Stivarga (regorafenib) EMA SmPC, January 2015.
Hector: Main Treatment-Emergent AEs by Cycle
Emergent AE
Treatment
G1 voice alteration
No dose modification
G1 ↑ amylase &
No dose modification
No dose modification
Treatment interruption for 1
week; treatment restarted with
dose reduction to 120 mg
Case courtesy of Dr Fortunato Ciardiello.
Which of the following tactics would you consider to manage a patient on regorafenib presenting with Grade 3 fatigue?
1. Inform patients about the potential benefits of exercise on
2. Monitor the patient weekly for the first 2 cycles and at every
visit (every 2 weeks) thereafter
3. Consider regorafenib dose interruption
4. Consider regorafenib dose reduction to 120 mg a day
5. All of the above
ASCO Practice Guidelines on Fatigue Management
• Cancer-related fatigue is one of the most prevalent, distressing, and activity-limiting symptoms
that patients experience, especially in the later stages of disease
• Fatigue is common side effect of cancer therapy
Address all medical and
Initiating/maintaining
treatable contributing
adequate levels of physical
factors (eg, pain, depression)
activity can reduce fatigue
Mind-Body
Cognitive behavioral and
Psychostimulants and other
psychoeducational therapies
wakefulness agents can be
can reduce fatigue
Mindfulness-based approaches,
used to manage fatigue
yoga, and acupuncture can
De Wit M, et al. Support Care Cancer. 2014;22(3):837-846 ; Bower JE, et al. J Clin Oncol. 2014;32(17):1840-1850.
Regorafenib AEs Occur Early in the First 8 Weeks of
Treatment and Stabilize or Decrease Over Time
Frequency of Common AEs (all grades) over Time*
*If patients experienced the same AE in more than 1 cycle, this is recorded separately for each of cycles 1-8.
Grothey A, et al. ASCO GI 2013. Abstract 467.
Hector: Tumor Assessment
Baseline CT
CT Scan #1
CT Scan #2
CT Scan #3
CT Scan #4
Regorafenib treatment summary:
December 2012–November 2013 (Cycle 13) with 1 dose level reduction (120 mg/d)
during cycle 5
Best response: SD
*Lung: lower right lobe.
‡Liver: multiple metastases.
Case courtesy of Dr Fortunato Ciardiello.
Effects on Tumor Size (CORRECT) and Rates of PR and SD
in CORRECT and CONCUR
Regorafenib
Best response, %
Complete response (CR)
Partial response (PR)
Stable disease (SD)
Progressive disease (PD)
*DCR, disease control rate.*DCR = PR + SD ≥6 weeks after randomization; P<.000001.
1. Van Cutsem E, et al. ESMO 2012. Abstract LBA18; Li J, et al. Lancet Oncol. 2015;16(6):619-629.
Hector: 72-Year-Old Man with Hypertension and Diabetes,
KRAS Wildtype Colon Cancer
Liver progression and
Progression of lung
new lung lesions
and liver metastases
PFS: 8 mo
PFS: 2 mo
Best response: SD
Case courtesy of Dr Fortunato Ciardiello.
• The patient was treated with 13 cycles of regorafenib and
• With efficient AE monitoring strategies, the patient had
longevity of treatment duration, despite comorbidities
• AEs were mild, with only 1 incidence of G3 fatigue, which
– With preventive measures using topical emollients, there was no
incidence of HFSR or rash
Case courtesy of Dr Fortunato Ciardiello.
Source: http://bkcmedia.tv/trm/3341/content/speaker2/slides2.pdf
Die Geburten meiner zwei Töchter – einmal ohne und einmal mit Traude. Kein Vergleich! BEL und Sophie Licht! Die Schwangerschaften Ich habe zwei gesunde Kinder (Sophie-Therese 2003, Amelie-Louise 2006) zur Welt gebracht und dennoch lässt sich dieses Kapitel ganz kurz halten: ich bin/war sehr gerne schwanger! Ich konnte es jedes Mal unheimlich genießen
How were new medicines discovered?David C. Swinney*‡ and Jason Anthony* Abstract Preclinical strategies that are used to identify potential drug candidates include target-based screening, phenotypic screening, modification of natural substances and biologic-based approaches. To investigate whether some strategies have been more successful than others in the discovery of new drugs, we analysed the discovery strategies and the molecular mechanism of action (MMOA) for new molecular entities and new biologics that were approved by the US Food and Drug Administration between 1999 and 2008. Out of the 259 agents that were approved, 75 were first-in-class drugs with new MMOAs, and out of these, 50 (67%) were small molecules and 25 (33%) were biologics. The results also show that the contribution of phenotypic screening to the discovery of first-in-class small-molecule drugs exceeded that of target-based approaches — with 28 and 17 of these drugs coming from the two approaches, respectively — in an era in which the major focus was on target-based approaches. We postulate that a target-centric approach for first-in-class drugs, without consideration of an optimal MMOA, may contribute to the current high attrition rates and low productivity in pharmaceutical research and development.












