
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Cathejell.ca
PRESCRIBING INFORMATION
PrCATHEJELL® Jelly 2%
(lidocaine hydrochloride jelly USP)
Topical Anesthetic
Pharmazeutische Fabrik Montavit Ges.m.b.H
Date of Preparation:
Salzbergstrasse 96
21 August 2014
6067 Absam, Austria
www.montavit.com
Distributed in Canada by:
BioSyent Pharma Inc.,
Toronto, Ontario,
Canada M9W 5Z5
Submission Control No: 176968
TABLE OF CONTENTS
PRESCRIBING INFORMATION . 1
TABLE OF CONTENTS . 2
PART I: HEALTH PROFESSIONAL INFORMATION . 3
SUMMARY PRODUCT INFORMATION . 3
INDICATIONS AND CLINICAL USE . 3
CONTRAINDICATIONS . 4
WARNINGS AND PRECAUTIONS . 4
ADVERSE REACTIONS . 7
DRUG INTERACTIONS . 8
DOSAGE AND ADMINISTRATION . 10
ACTION AND CLINICAL PHARMACOLOGY . 13
STORAGE AND STABILITY . 15
SPECIAL HANDLING INSTRUCTIONS . 15
DOSAGE FORMS, COMPOSITION AND PACKAGING . 16
PART III: CONSUMER INFORMATION . 17
PrCATHEJELL® Jelly 2%
(lidocaine hydrochloride jelly USP)
PART I: HEALTH PROFESSIONAL INFORMATION
SUMMARY PRODUCT INFORMATION
Dosage Form / Strength
Non-medicinal Ingredients
Administration
Jelly in collapsible syringe 20 mg/g (When fully compressed each syringe
glycerol, hydrochloric acid,
expresses approximately
hydroxyethylcellulose, sodium hydroxide, and
10.0g (corr. to 9.4 mL,
water for injection
200 mg of lidocaine hydrochloride)
INDICATIONS AND CLINICAL USE Adults (>18 years of age):
CATHEJELL Jelly 2% (lidocaine hydrochloride) is indicated for:
Surface anesthesia and lubrication for:
•
The male and female urethra during cystoscopy, catheterization, exploration by sound
and other endourethral operations;
Nasal and pharyngeal cavities in endoscopic procedures such as gastroscopy and bronchoscopy;
Proctoscopy and rectoscopy;
Tracheal intubation.
Symptomatic treatment of pain in connection with cystitis and urethritis.
Geriatrics (> 65 years of age): Elderly patients should be given reduced doses commensurate with their age and physical
condition (see DOSAGE AND ADMINISTRATION-Special Populations).
Pediatrics (<18 years of age): Children should be given reduced doses commensurate with their age, weight and physical
condition (see DOSAGE AND ADMINISTRATION-Special Populations).
Lidocaine should be used with caution in children younger than two years of age as there are
insufficient data to support the safety and efficacy of this product in this patient population at this
time (see WARNINGS AND PRECAUTIONS-Special Populations).
CATHEJELL Jelly 2% (lidocaine hydrochloride) is contraindicated in: •
patients with a known history of hypersensitivity to local anesthetics of the amide type or to other components in the formulation (see DOSAGE FORMS, COMPOSITION AND PACKAGING).
WARNINGS AND PRECAUTIONS
EXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS OF LIDOCAINE OR ITS METABOLITES AND SERIOUS ADVERSE EFFECTS. Absorption from the mucous membranes is variable but is especially high from the bronchial tree. Such applications may therefore result in rapidly rising or excessive plasma concentrations, with an increased risk for toxic symptoms, such as convulsions. PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE. This is especially important in children where doses vary with weight. The management of serious adverse reactions may require the use of resuscitative equipment, oxygen and other resuscitative drugs (see OVERDOSAGE). The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Tolerance to elevated blood levels varies with the status of the patient. Lidocaine should be used with caution in patients with sepsis and/or traumatized mucosa at the area of application, since under such conditions there is the potential for rapid systemic absorption. CATHEJELL Jelly 2% should be used with caution in children under the age of 2 as there is insufficient data to support the safety and efficacy of this product in this patient population at this time. In patients under general anesthesia who are paralyzed, higher plasma concentrations may occur than in spontaneously breathing patients. Unparalyzed patients are more likely to swallow a large proportion of the dose, which then undergoes considerable first-pass hepatic metabolism following absorption from the gut. Avoid contact with eyes.
Many drugs used during the conduct of anesthesia are considered potential triggering agents for
familial malignant hyperthermia. It has been shown that the use of amide local anesthetics in
malignant hyperthermia patients is safe. However, there is no guarantee that neural blockade
will prevent the development of malignant hyperthermia during surgery. It is also difficult to
predict the need for supplemental general anesthesia. Therefore, a standard protocol for the
management of malignant hyperthermia should be available.
When used for endotracheal tube lubrication, care should be taken to avoid introduction of the
jelly into the lumen of the tube. If allowed into the inner lumen, the jelly may dry on the inner
surface leaving a residue which tends to clump with flexion, narrowing the lumen. There have
been rare reports in which this residue has caused the lumen to occlude. Similarly, do not use the
jelly to lubricate the endotracheal stylettes.
When topical anesthetics are used in the mouth, the patient should be aware that the production
of topical anesthesia may impair swallowing and thus enhance the danger of aspiration.
Numbness of the tongue or buccal mucosa may enhance the danger of unintentional biting
trauma. Food or chewing gum should not be taken while the mouth or throat area is
anesthetized. See also Part III: Consumer Information.
CATHEJELL Jelly 2% is ineffective when applied to intact skin.
Lidocaine has been shown to be porphyrinogenic in animal models. CATHEJELL Jelly 2%
should only be prescribed to patients with acute porphyria on strong or urgent indications, when
they can be closely monitored. Appropriate precautions should be taken for all porphyric
patients.
Cardiovascular
Lidocaine should be used with caution in patients with bradycardia or impaired cardiovascular
function since they may be less able to compensate for functional changes associated with the
prolongation of A-V conduction produced by amid-type local anesthetics.
Lidocaine should be used with caution in patients in severe shock.
Hepatic
Because amide-type local anesthetics such as lidocaine are metabolized by the liver, these drugs,
especially repeated doses, should be used cautiously in patients with hepatic disease. Patients
with severe hepatic disease, because of their inability to metabolize local anesthetics normally,
are at greater risk of developing toxic plasma concentrations.
Neurologic
Epilepsy: The risk of central nervous system side effects when using lidocaine in patients with
epilepsy is very low, provided that the dose recommendations are followed. (See DOSAGE
AND ADMISTRATION).
Locomotion and Coordination: Topical lidocaine formulations generally result in low plasma
concentrations because of a low degree of systemic absorption. However, depending on the dose,
local anesthetics may have a very mild effect on mental function and coordination even in the
absence of overt CNS toxicity and may temporarily impair locomotion and alertness.
Renal
Lidocaine is metabolized primarily by the liver to monoethylglycinexylidine (MEGX, which has
some CNS activity), and then further to metabolites glycinexylidine (GX) and 2,6dimethylaniline
(see ACTION AND CLINICAL PHARMACOLOGY). Only a small fraction (2%) of lidocaine
is excreted unchanged in the urine. The pharmacokinetics of lidocaine and its main metabolite
were not altered significantly in haemodialysis patients (n=4) who received an intravenous dose
of lidocaine. Therefore, renal impairment is not expected to significantly affect the
pharmacokinetics of lidocaine when CATHEJELL Jelly 2% is used for short treatment durations,
according to dosage instructions (see DOSAGE AND ADMINISTRATION). Caution is
recommended when lidocaine is used in patients with severely impaired renal function because
lidocaine metabolites may accumulate during long term treatment (see DOSAGE AND
ADMINISTRATION).
Sensitivity
Lidocaine should be used with caution in persons with known drug sensitivities.
CATHEJELL Jelly 2% is contraindicated in patients with known hypersensitivities to local
anesthetics of the amide type, and to other components in the formulation.
Special Populations
Debilitated patients, acutely ill patients, and patients with sepsis should be given reduced doses
commensurate with their age, weight and physical condition because they may be more sensitive
to systemic effects due to increased blood levels of lidocaine following repeated doses.
Pregnant Women: There are no adequate and well-controlled studies in pregnant women on the
effect of lidocaine on the developing fetus.
It is reasonable to assume that a large number of pregnant women and women of child-bearing
age have been given lidocaine. No specific disturbances to the reproductive process have so far
been reported, e.g. no increased incidence of malformations. However, care should be given
during early pregnancy when maximum organogenesis takes place.
Labour and Delivery: Lidocaine is not contraindicated in labour and delivery. Should
CATHEJELL Jelly 2% be used concomitantly with other products containing lidocaine during
labour and delivery, the total dose contributed by all formulations must be kept in mind.
Nursing Women: Lidocaine and its metabolites are excreted in the breast milk. At therapeutic
doses, the quantities of lidocaine and its metabolites in breast milk are small and generally are
not expected to be a risk for the infant.
Pediatrics: Children should be given reduced doses commensurate with their age, weight and
physical condition because they may be more sensitive to systemic effects due to increased blood
levels of lidocaine following repeated doses (see DOSAGE AND ADMINISTRATION).
CATHEJELL Jelly 2% should be used with caution in children under the age of 2 as there is
insufficient data to support the safety and efficacy of this product in this patient population at this
time.
Geriatrics: Elderly patients may be more sensitive to systemic effects due to increased blood
levels of lidocaine following repeated doses and may require dose reductions.
Carcinogenesis and Mutagenesis
Genotoxicity tests with lidocaine showed no evidence of mutagenic potential. A metabolite of
lidocaine, 2,6-dimethylaniline, showed weak evidence of activity in some genotoxicity tests. A
chronic oral toxicity study of the metabolite 2,6-dimethylaniline (0, 14, 45, 135 mg/kg)
administered in feed to rats showed that there was a significantly greater incidence of nasal
cavity tumors in male and female animals that had daily oral exposure to the highest dose of 2,6-
dimethylaniline for 2 years. The lowest tumor-inducing dose tested in animals (135 mg/kg)
corresponds to approximately 50 times the amount of 2,6-dimethylaniline to which a 50 kg
subject would be exposed following the application of 20 g of lidocaine jelly 2% for 24 hours on
the mucosa, assuming the highest theoretical extent of absorption of 100% and 80% conversion
to 2,6-dimethylaniline. Based on a yearly exposure (once daily dosing with 2,6dimethylaniline in
animals and 5 treatment sessions with 20 g lidocaine jelly 2% in humans), the safety margins
would be approximately 3400 times when comparing the exposure in animals to man.
ADVERSE REACTIONS
Adverse experiences following the administration of lidocaine are similar in nature to those
observed with other amide local anesthetic agents. These adverse experiences are, in general,
dose-related and may result from high plasma levels caused by overdosage or rapid absorption,
or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the
patient.
An increased incidence of postoperative sore throat has been reported following endotracheal
tube lubrication with lidocaine jelly.
Serious adverse experiences are generally systemic in nature. The following types are those
most commonly reported:
Central Nervous System: CNS manifestations are excitatory and/or depressant and may be
characterized by the following signs and symptoms of escalating severity: circumoral
paresthesia, light-headedness, nervousness, apprehension, euphoria, confusion, dizziness,
drowsiness, hyperacusis, tinnitus, blurred vision, vomiting, sensations of heat, cold or numbness,
twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The
excitatory manifestations (e.g., twitching, tremors, convulsions) may be very brief or may not
occur at all, in which case the first manifestation of toxicity may be drowsiness merging into
unconsciousness and respiratory arrest.
Drowsiness following the administration of lidocaine is usually an early sign of a high lidocaine
plasma level and may occur as a consequence of rapid absorption.
Cardiovascular System: Cardiovascular manifestations are usually depressant and are
characterized by bradycardia, hypotension, arrhythmia and cardiovascular collapse, which may
lead to cardiac arrest.
Allergic: Allergic reactions are characterized by cutaneous lesions, urticaria, edema or, in the
most severe instances, anaphylactic shock. Allergic reactions of the amide type are rare (<0.1%)
and may occur as a result of sensitivity either to the local anesthetic agent or to other components
in the formulation (see DOSAGE FORM, COMPOSITION AND PACKAGING).
DRUG INTERACTIONS
Overview
Lidocaine is mainly metabolized in the liver by CYP1A2 and CYP3A4 to its two major
metabolites, monoethylglycinexylidine (MEGX) and glycinexylidine (GX), both of which are
pharmacologically active. Lidocaine has a high hepatic extraction ratio. Only a small fraction
(2%) of lidocaine is excreted unchanged in the urine. The hepatic clearance of lidocaine is
expected to depend largely on blood flow.
Strong inhibitors of CYP1A2, such as fluvoxamine, given concomitantly with lidocaine, can
cause a metabolic interaction leading to an increased lidocaine plasma concentration.
Therefore, prolonged administration of lidocaine should be avoided in patients treated with
strong inhibitors of CYP1A2, such as fluvoxamine. When co-administered with intravenous
lidocaine, two strong inhibitors of CYP3A4, erythromycin and itraconazole, have each been
shown to have a modest effect on the pharmacokinetics of intravenous lidocaine. Other drugs
such as propranolol and cimetidine have been reported to reduce intravenous lidocaine clearance,
probably through effects on hepatic blood flow and/or metabolism.
When lidocaine is used topically, plasma concentrations are of importance for safety reasons (see
WARNINGS AND PRECAUTIONS, General; ADVERSE REACTIONS). However, with the
low systemic exposure and short duration of topical application, the abovementioned metabolic
drug-drug interactions are not expected to be of clinical significance when CATHEJELL Jelly
2% is used according to dosage recommendations.
Clinically relevant pharmacodynamic drug interactions may occur with lidocaine and other local
anesthetics or structurally related drugs, and Class I and Class III antiarrhythmic drugs due to
additive effects.
Drug-Drug Interactions
Local anesthetics and agents structurally related to amide-type local anesthetics
Lidocaine should be used with caution in patients receiving other local anesthetics or agents
structurally related to amide-type local anesthetics (e.g. antiarrhythmics such as mexiletine),
since the toxic effects are additive.
Antiarryhythmic Drugs
Class I Antiarrhythmic drugs
Class I antiarrhythmic drugs (such as mexiletine) should be used with caution since toxic effects
are additive and potentially synergistic.
Class III Antiarrhythmic drugs
Caution is advised when using Class III antiarrhythmic drugs concomitantly with lidocaine due
to potential pharmacodynamic or pharmacokinetic interactions with lidocaine, or both. A drug
interaction study has shown that the plasma concentration of lidocaine may be increased
following administration of a therapeutic dose of intravenous lidocaine to patients treated with
amiodarone (n=6). Case reports have described toxicity in patients treated concomitantly with
lidocaine and amiodarone. Patients treated with Class III antiarrhythmic drugs (e.g. amiodarone)
should be kept under close surveillance and ECG monitoring should be considered, since cardiac
effects of these drugs and lidocaine may be additive.
Strong Inhibitors of CYP1A2 and CYP3A4
Cytochrome CYP1A2 and CYP3A4 are involved in the formation of the pharmacologically active lidocaine metabolite MEGX.
Fluvoxamine: Strong inhibitors of CYP1A2, such as fluvoxamine, given during prolonged administration of lidocaine to areas with a high extent of systemic absorption (e.g., mucous membranes) can cause a metabolic interaction leading to an increased lidocaine plasma concentration. The plasma clearance of a single intravenous dose of lidocaine was reduced by 41 to 60% during co-administration of fluvoxamine, a selective and potent CYP1A2 inhibitor, to healthy volunteers.
Erythromycin and Itraconazole: Erythromycin and itraconazole, which are strong inhibitors of CYP3A4, have been shown to reduce clearance of lidocaine by 9 to 18%, following a single intravenous dose of lidocaine to healthy volunteers. During combined co-administration with fluvoxamine and erythromycin the plasma clearance of lidocaine was reduced by 53%. β-blockers and cimetidine
Following a single intravenous dose of lidocaine, administered to healthy volunteers, the
clearance of lidocaine has been reported to be reduced up to 47% when co-administered with
propanolol and up to 30% when co-administered with cimetidine. Reduced clearance of
lidocaine when co-administered with these drugs is probably due to reduced liver blood flow
and/or inhibition of microsomal liver enzymes. The potential for clinically significant
interactions with these drugs should be considered during long-term treatment with high doses of
lidocaine.
Drug-Food Interactions
Interactions of lidocaine with food have not been established.
Drug-Herb Interactions
Interactions of lidocaine with herbal products have not been established.
Drug-Laboratory Tests Interactions
Interactions of lidocaine with laboratory tests have not been established.
Drug-Lifestyle Interactions
Interactions of lidocaine with lifestyle have not been established.
DOSAGE AND ADMINISTRATION
Dosing Considerations
When CATHEJELL Jelly 2% (lidocaine hydrochloride) is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind.
• CATHEJELL Jelly 2% in the plastic syringe is preservative-free, and intended for single
The absorption of lidocaine jelly from the nasopharynx is usually lower than with other lidocaine
products. Blood concentrations of lidocaine after instillation of the jelly in the intact urethra and
bladder in doses up to 800 mg are fairly low and below toxic levels.
Special Populations
Lidocaine should also be used with caution in patients with epilepsy, impaired cardiac
conduction, bradycardia, impaired hepatic or renal function and in severe shock (see
WARNINGS AND PRECAUTIONS).
Debilitated patients, elderly patients, acutely ill patients, patients with sepsis, and children should
be given reduced doses commensurate with their age, weight and physical condition (see
WARNINGS AND PRECAUTIONS).
CATHEJELL Jelly 2% should be used with caution in children under the age of 2 as there is
insufficient data to support the safety and efficacy of this product in this patient population at this
time (see WARNINGS AND PRECAUTIONS).
Recommended Dose and Dosage Adjustment
When fully compressed each 1 x 12.5 g syringe will express approximately 10.0 g (corr. to 9.4 mL)
of CATHEJELL Jelly 2% (200 mg of lidocaine hydrochloride).
Urethral Anesthesia
Surface Anesthesia of the Male Adult Urethra
For adequate analgesia in males, 20 g (400 mg lidocaine hydrochloride) jelly (2 syringes) is
usually required. The jelly is instilled slowly until the patient has a feeling of tension,
approximately 10 g (200 mg lidocaine hydrochloride) jelly (1 syringe). A penile clamp is then
applied for several minutes at the corona, after which another 10 g (200 mg lidocaine
hydrochloride) of the jelly (1 syringe) is instilled.
When anesthesia is especially important, e.g., during sounding or cystoscopy, a larger quantity of
jelly (e.g., 30-40 g, i.e., 600-800 mg lidocaine hydrochloride) (3-4 syringes) may be instilled in
3-4 portions and allowed to act for 10-12 minutes before insertion of the instrument. The jelly
instilled into the bladder is also effective for procedures in this region.
To anesthetize only the anterior male urethra, e.g., for catheterization, small volumes (e.g. 5-10 g,
i.e., 100-200 mg lidocaine hydrochloride) (½ to 1 syringe) are usually adequate for lubrication.
For Surface Anesthesia of the Female Adult Urethra
Instill 5-10 g (100-200 mg lidocaine hydrochloride) jelly (½ to 1 syringe) in small portions to fill
the whole urethra. If desired, some jelly may be deposited on the orifice and covered with a
cotton swab. In order to obtain adequate anesthesia, several minutes should be allowed prior to
performing urological procedures.
Endoscopy
The instillation of 10-20 g (200-400 mg lidocaine hydrochloride) jelly (1-2 syringes) is
recommended for adequate analgesia and a small amount may be applied to the lubricating
instrument. When combined with other lidocaine products (e.g., for bronchoscopy), the total
dose of lidocaine hydrochloride should not exceed 400 mg (20 g jelly) (2 syringes).
Proctoscopy and rectoscopy
Up to 20 g (400 mg lidocaine hydrochloride) jelly (2 syringes) can be used for anal and rectal
procedures. The total dose of lidocaine hydrochloride should not exceed 400 mg (20 g jelly) (2
syringes).
Lubrication for Endotracheal Intubation
Apply approximately 2 g of jelly to the external surface of the endotracheal tube just prior to
insertion. Care should be taken to avoid introducing the product into the lumen of the tube (see
WARNINGS AND PRECAUTIONS). Do not use the jelly to lubricate endotracheal stylettes. It
is also recommended that the use of endotracheal tubes with dried jelly on the external surface be
avoided for lack of lubricating effect.
Maximum Dosage
The dose of CATHEJELL Jelly 2% depends on the application site. A safe dose for oral use is 400 mg lidocaine hydrochloride (20 g jelly) (2 syringes). A safe dose for use in the urethra and
bladder is 800 mg lidocaine hydrochloride (40 g jelly) (4 syringes). A maximum single dosage
for CATHEJELL Jelly 2% is not established. No more than four doses should be given during a
24-hour period.
Children (Under 12 Years)
It is difficult to recommend a maximum dose of any drug for children since this varies as a
function of age and weight. The maximum amount per dose of CATHEJELL Jelly 2% should not
exceed 6 mg/kg of body weight or 3 g CATHEJELL Jelly 2% per 10 kg body weight. No more
than four doses should be given during a 24-hour period.
For children over 12 years of age doses should be commensurate with weight and physical
condition.
OVERDOSAGE
For management of suspected drug overdose, contact your regional Poison Control Centre.
Acute systemic toxicity from local anesthetics is generally related to high plasma levels
encountered during therapeutic use of local anesthetics and originates mainly in the central
nervous and the cardiovascular systems (see ADVERSE REACTIONS and WARNINGS AND
PRECAUTIONS). It should be kept in mind that clinically relevant pharmacodynamic drug
interactions (i.e., toxic effects) may occur with lidocaine and other local anesthetics or
structurally related drugs, and Class I and Class III antiarrhythimic drugs due to additive effects
(see DRUG INTERACTIONS).
Symptoms
Central nervous system toxicity is a graded response, with symptoms and signs of escalating
severity. The first symptoms are circumoral paresthesia, numbness of the tongue,
lightheadedness, hyperacusis and tinnitus. Visual disturbance and muscular tremors are more
serious and precede the onset of generalized convulsions. Unconsciousness and grand mal
convulsions may follow, which may last from a few seconds to several minutes. Hypoxia and
hypercarbia occur rapidly following convulsions due to the increased muscular activity, together
with the interference with normal respiration. In severe cases apnea may occur. Acidosis,
hyperkalaemia, hypocalcaemia and hypoxia increase and extend the toxic effects of local
anesthetics.
Recovery is due to redistribution and metabolism of the local anesthetic drug. Recovery may be
rapid unless large amounts of the drug have been administered.
Cardiovascular effects may be seen in cases with high systemic concentrations. Severe
hypotension, bradycardia, arrhythmia and cardiovascular collapse may be the result in such
cases.
Cardiovascular toxic effects are generally preceded by signs of toxicity in the central nervous
system, unless the patient is receiving a general anesthetic or is heavily sedated with drugs such
as a benzodiazepine or barbiturate.
Treatment
The first consideration is prevention, best accomplished by careful and constant monitoring of
cardiovascular and respiratory vital signs and the patient's state of consciousness after each local
anesthetic administration. At the first sign of change, oxygen should be administered.
The first step in the management of systemic toxic reactions consists of immediate attention to
the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a
delivery system capable of permitting immediate positive airway pressure by mask. This may
prevent convulsions if they have not already occurred.
If convulsions occur, the objective of the treatment is to maintain ventilation and oxygenation
and support circulation. Oxygen must be given and ventilation assisted if necessary (mask and
bag or tracheal intubation). Should convulsions not stop spontaneously after 15-20 seconds, an
anticonvulsant should be given iv to facilitate adequate ventilation and oxygenation. Thiopental
sodium 1-3 mg/kg iv is the first choice. Alternatively diazepam 0.1 mg/kg bw iv may be used,
although its action will be slow. Prolonged convulsions may jeopardise the patient's ventilation
and oxygenation. If so, injection of a muscle relaxant (e.g. succinylcholine 1 mg/kg bw) will
facilitate ventilation, and oxygenation can be controlled. Early endotracheal intubation is
required when succinylcholine is used to control motor seizure activity.
If cardiovascular depression is evident (hypotension, bradycardia), ephedrine 5-10 mg i.v. should
be given and may be repeated, if necessary, after 2-3 minutes.
Should circulatory arrest occur, immediate cardiopulmonary resuscitation should be instituted.
Continual oxygenation and ventilation and circulatory support as well as treatment of acidosis
are of vital importance, since hypoxia and acidosis will increase the systemic toxicity of local
anesthetics. Epinephrine (0.1-0.2 mg as intravenous or intracardial injections) should be given as
soon as possible and repeated, if necessary.
Children should be given doses of epinephrine commensurate with their age and weight.
ACTION AND CLINICAL PHARMACOLOGY
Mechanism of Action
Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the
initiation and conduction of impulses, thereby effecting local anesthetic action. Local anesthetics
of the amide type are thought to act within the sodium channels of the nerve membrane.
Onset of Action
Anesthesia is achieved within 5 minutes, depending on the area of application. Duration of
anesthesia is approximately 20-30 minutes. CATHEJELL Jelly 2% (lidocaine hydrochloride) is
ineffective when applied to intact skin.
Hemodynamics
Lidocaine, like other local anesthetics, may also have effects on excitable membranes in the
brain and myocardium. If excessive amounts of drug reach systemic circulation rapidly,
symptoms and signs of toxicity will appear, emanating from the central nervous and
cardiovascular systems.
Central nervous system toxicity (see OVERDOSAGE) usually precedes the cardiovascular
effects since it occurs at lower plasma concentrations. Direct effects of local anesthetics on the
heart include slow conduction, negative inotropism and eventually cardiac arrest.
Pharmacokinetics
Absorption: The rate and extent of absorption depends upon concentration and total dose
administered, the specific site of application and duration of exposure. In general, the rate of
absorption of local anesthetic agents following topical application to wound surfaces and mucous
membranes is high, and occurs most rapidly after intratracheal and bronchial administration.
The absorption of lidocaine jelly from the nasopharynx is usually lower than with other lidocaine
products. Blood concentrations of lidocaine after instillation of the jelly in the intact urethra and
bladder in doses up to 800 mg are fairly low and below toxic levels. Lidocaine is also well
absorbed from the gastrointestinal tract, although little intact drug may appear in the circulation
because of biotransformation in the liver.
Distribution: Lidocaine has a total plasma clearance of 0.95 L/min and a volume of distribution
at steady state of 91 L.
Lidocaine readily crosses the placenta, and equilibrium in regard to free, unbound drug will be
reached. Because the degree of plasma protein binding in the fetus is less than in the mother, the
total plasma concentration will be greater in the mother, but the free concentrations will be the
same.
The plasma binding of lidocaine is dependent on drug concentration, and the fraction bound
decreases with increasing concentration. At concentrations of 1 to 4 µg of free base per mL, 60
to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma
concentration of the alpha-1-acid glycoprotein. Lidocaine crosses the blood-brain and placental
barriers, presumably by passive diffusion.
Metabolism: Lidocaine is metabolized rapidly by the liver, and its metabolites and the
unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-
dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. Only 2% of
lidocaine is excreted unchanged. Most of it is metabolized first to monoethylglycinexylidide
(MEGX) and then to glycinexylidide (GX) and 2,6-dimethylaniline. Up to 70% appears in the
urine as 4-hydroxy- 2,6-dimethylaniline. The pharmacological/toxicological actions of MEGX
and GX are similar to, but less potent than those of lidocaine. GX has a longer half-life (about
10 h) than lidocaine and may accumulate during long-term administration.
Excretion: Lidocaine has an elimination half-life of 1.6 h and an estimated hepatic extraction
ratio of 0.65. The clearance of lidocaine is almost entirely due to liver metabolism, and depends
both on liver blood flow and the activity of metabolizing enzymes. Approximately 90% of the
lidocaine administrated intravenously is excreted in the form of various metabolites, and less
than 10% is excreted unchanged in the urine. The primary metabolite in urine is a conjugate of
4-hydroxy- 2,6-dimethylaniline, accounting for about 70-80% of the dose excreted in the urine.
The elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to
2.0 hours. The elimination half-life in neonates (3.2 h) is approximately twice that of adults. The
half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal
dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites.
Special Populations and Conditions
Acidosis increases the systemic toxicity of lidocaine while the use of CNS depressants may
increase the levels of lidocaine required to produce overt CNS effects. Objective adverse
manifestations become increasingly apparent with increasing venous plasma levels above 6.0 µg
free base per mL.
STORAGE AND STABILITY
Store at 15-30°C.
SPECIAL HANDLING INSTRUCTIONS
1.
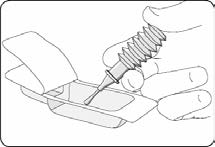
Clean and disinfect the affected area if possible.
Peel off the paper cover from the transparent blister pack. Inspect mixture visually for
clarity, particulate matter, precipitation, discolouration and leakage prior to administration.
Break off the applicator tip in the blister pack.
Remove the applicator tip completely.
Release one drop of the jelly to coat the nozzle for easier insertion.
Instillation is completed by applying slight but steady pressure to the collapsible syringe.
To avoid suction, keep the collapsible syringe compressed whilst removing from the
For further assistance please contact Pharmazeutische Fabrik MONTAVIT Ges.m.b.H. at or BioSyent Pharma Inc., at, Tel: 1-888-439-0013
DOSAGE FORMS, COMPOSITION AND PACKAGING
Dosage Forms
CATHEJELL Jelly 2% (lidocaine hydrochloride) is a clear to almost clear, slightly coloured
jelly.
Composition
When fully compressed each 1 x 12.5 g syringe will express approximately 10.0 g (corr. to 9.4 mL)
of CATHEJELL Jelly 2% (200 mg of lidocaine hydrochloride).
Non-medicinal ingredients:
glycerol hydrochloric acid hydroxyethylcellulose
sodium hydroxide water for injection
The jelly syringe contains no preservatives and is intended for single use only.
Packaging
CATHEJELL Jelly 2% is available in collapsible syringes (accordion-type syringes) with applicator
cone, both of polypropylene . Packaging sizes include 1 x 12.5g, 5 x 12.5 g, 25 x 12.5 g.
IMPORTANT: PLEASE READ
CATHEJELLTM Jelly 2%
WHAT THE MEDICINAL INGREDIENT IS:
lidocaine hydrochloride jelly USP
lidocaine hydrochloride 2%
PART III:
NONMEDICINAL INGREDIENTS
CATHEJELL Jelly 2% also contains glycerol, hydrochloric
CONSUMER INFORMATION
acid, hydroxyethylcellulose, sodium hydroxide and water for
This leaflet is part III of a two-part "Prescribing
Information" published when CATHEJELL Jelly 2%
was approved for sale in Canada and is designed
Tell your doctor if you think you may be sensitive to any of
specifically for Consumers. This leaflet is a summary and
the above ingredients.
will not tell you everything about CATHEJELL Jelly
2%. Contact your doctor or pharmacist if you have any
WHAT DOSAGE FORMS IT COMES IN
questions about the drug.
CATHEJELL Jelly 2% comes in a single use syringe size of
20 mg lidocaine hydrochloride/g of jelly.
Before using CATHEJELL Jelly 2%, read this leaflet
carefully.
WARNINGS AND PRECAUTIONS
Please keep this leaflet to refer to until you have used up
BEFORE you use CATHEJELL Jelly 2% tell your doctor or
all your CATHEJELL Jelly 2%.
• about all health problems you have now or have had in
This medicine has been prescribed for you personally
and you should not pass it on to others. It may harm
• about other medicines you take, including ones you can
them, even if their symptoms are the same as yours.
buy without a prescription;
• if you are taking other medicines such as drugs used to
ABOUT THIS MEDICATION
treat irregular heart activity (anti-arrhythmics);
• if you have ever had a bad, unusual or allergic reaction
WHAT THE MEDICATION IS USED FOR:
to CATHEJELL Jelly 2% or any other medicines ending
CATHEJELL Jelly 2% is used to produce a temporary loss
with "caine";
of feeling or numbness of the skin in adults and children 2
• if you think you may be allergic or sensitive to any
years of age and older, and can be used:
ingredients in CATHEJELL Jelly 2% (see above);
if there is an infection, skin rash, cut or wound at or near
before certain types of examinations done by your
the area you want to apply CATHEJELL Jelly 2%;
if you have a skin condition that is severe or that covers
to help relieve the pain from inflammation of the urinary
bladder and the urethra
• if you have a severe heart, kidney or liver disease (see
PROPER USE OF THIS MEDICATION section);
WHAT IT DOES:
• if you have epilepsy (there is very low risk if used as per
CATHEJELL Jelly 2% is the brand name for a topical
PROPER USE OF THIS MEDICATION section);
anesthetic that contains the drug lidocaine. Topical
anesthetics are used to produce a temporary loss of sensation
If you or someone in your family has been diagnosed
or numbness on the area where they are applied.
if you are experiencing severe shock;
CATHEJELL Jelly 2% should start to work within 5 to 15
if you are pregnant, plan to become pregnant or are
minutes after you apply it. The effect usually lasts 20 to 30
INTERACTIONS WITH THIS MEDICATION
WHEN IT SHOULD NOT BE USED
Do not use CATHEJELL Jelly 2% if you:
Tell your doctor or pharmacist about any other drugs you
take, including:
• are allergic to lidocaine, any other "-caine" type
• drugs you can buy without a prescription
anesthetics (for example, bupivacaine, ropivacaine or
• anti-arrhythmic drugs for heart problems (e.g.
mepivacaine), or any of the non-medicinal ingredients in
mexiletine, amiodarone) (see PROPER USE OF THIS
the product (see
NONMEDICINAL INGREDIENTS
MEDICATION section);
• other anesthetics (see PROPER USE OF THIS
MEDICATION section);
IMPORTANT: PLEASE READ
• propranolol for heart problems or cimetidine for
Dose for Adults:
gastrointestinal problems, if you are going to use high
The dose of the jelly depends on the application site. For
doses of CATHEJELL Jelly 2% for a long time;
oral use of CATHEJELL Jelly 2% a dose of 20 g (2
• fluvoxamine, for depression, if you are going to use
syringes) is usually safe. For use in the urethra (i.e., before
high doses of CATHEJELL Jelly 2% for a long time.
insertion of urinary catheters or urinary procedures), 5 to
20 g (½ to 2 syringes) is usually enough. A safe dose for use
Please inform your doctor/dentist/pharmacist if you are
in the urethra and bladder is 40 g (4 syringes).
taking or have recently taken any other medicines, even
those that can be bought without a prescription. Usage of
When fully compressed each 1 x 12.5 g syringe will express
such medicines at the same time may increase the risk of
approximately 10 g (9.4 mL) of CATHEJELL Jelly 2%
serious side effects.
(200 mg of lidocaine hydrochloride).
PROPER USE OF THIS MEDICATION
No more than four doses should be given during a 24-hour
USUAL DOSE:
If this medicine is recommended by your doctor, be sure to
Dose for Children Under 12 Years of Age:
follow the directions for use that have been given. If you are
The dose depends on the child's weight. No more than 3 g of
treating yourself, such as for the treatment of pain caused by
jelly per 10 kilograms of the child's weight should be used
inflammation of the urethra or urinary bladder, or for self
per dose. For a 10 kg child the dose should be no more than
catheterization, follow the directions below and your
1/3 of the tube.
doctor's or pharmacist's instructions for how to apply the
jelly. Check with your doctor or pharmacist if you have any
No more than four doses should be given during a 24-hour
questions about your directions.
The following are general guidelines for the maximum
• For use in children under 2 years of age, consult a
amount of CATHEJELL Jelly 2% that should be used
without a doctor's advice. These guidelines apply only to
• If you have any questions about how to measure the
otherwise healthy people. If you have a special skin or other
above amounts, be sure to ask your pharmacist.
condition that requires a doctor's supervision ask your
• If you are treating yourself and your condition does
doctor about the maximum amount of jelly that you should
not seem to improve within three to five days,
check with your doctor about continuing to use
CATHEJELL Jelly 2%.
Do not use more CATHEJELL Jelly 2%; or more often or
for a longer period of time than either your doctor ordered or
Instructions for Use – Jelly Syringe
than these package directions suggest as this may cause
Clean and disinfect the affected area if possible.
unwanted side effects (see SERIOUS SIDE EFFECTS,
Peel off the paper cover from the transparent blister
HOW OFTEN THEY HAPPEN AND WHAT TO DO
pack. Inspect jelly visually for clarity, particulate
matter, precipitation, discolouration, and leakage prior
to administration.
• If possible, clean the affected area well, before each
application of jelly.
• Use the smallest amount of jelly needed to control your
• Avoid contact with your eyes.
Conditions where dose adjustments may be required:
• elderly patients
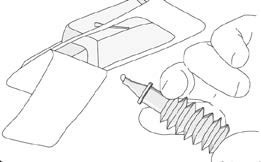
Break off the applicator tip in the blister pack.
• acutely ill patients
• patients with severe liver disease • patients with severe kidney disease
• patients also treated with other anesthetics or certain
antiarrhythmic drugs (such as amiodarone or mexiletine)
IMPORTANT: PLEASE READ
Remove the applicator tip completely.
mean that you will get them. If any side effects bother you,
Release one drop of the jelly to coat the nozzle for
or if you experience any unusual effects while you are using
easier insertion.
CATHEJELL Jelly 2% stop using it and check with your
doctor or pharmacist as soon as possible.
SERIOUS SIDE EFFECTS, HOW OFTEN THEY
HAPPEN AND WHAT TO DO ABOUT THEM
Symptom / effect
Talk with your
Stop taking
doctor or
pharmacist
immediate
Instillation is completed by applying slight but steady
emergency
pressure to the collapsible syringe.
To avoid suction, keep the collapsible syringe
attention.
compressed whilst removing from the affected area.
Allergic
reaction such
as: redness,
OVERDOSE:
For management of suspected drug overdose, contact your
regional Poison Control Centre.
Early signs of overdosage are numbness of the lips and
around the mouth, lightheadedness, dizziness and sometimes
blurred vision. In the event of a serious overdosage,
trembling, seizures or unconsciousness may occur.
problems, swelling of
If the early signs of overdosage are noticed and no further
CATHEJELL Jelly 2% is given, the risk of serious adverse
effects occurring rapidly decreases. If you think you or
anyone else is experiencing any of the above signs,
telephone your doctor or go to the nearest hospital right
Overdose as
SIDE EFFECTS AND WHAT TO DO ABOUT THEM
Like any medication, CATHEJELL Jelly 2% may cause side
effects in some people.
Avoid eating or chewing gum when CATHEJELL Jelly 2%
is used in the mouth or throat since numbness in these areas
may interfere with swallowing and could potentially cause
choking. Numbness of the tongue or gums may also increase
the danger of injury due to biting.
Avoid exposure to extreme hot or cold temperatures (e.g.
food, drink) until complete sensation has returned.
Avoid contact with the eyes because numbness in the eyes
may prevent you from noticing if you get something in your
With the recommended doses, CATHEJELL Jelly 2% has no
effect on the ability to drive and use machines.
This is not a complete list of side effects. For any
Medicines affect different people in different ways. Just
unexpected effects while taking CATHEJELL Jelly 2%
because side effects have occurred in some patients, does not
contact your doctor or pharmacist.
IMPORTANT: PLEASE READ
The above side effects are extremely rare, but can occur
For the most current information, the Consumer Information
when too much CATHEJELL Jelly 2% is used at one time
Leaflet plus the full Prescribing Information, prepared for
and when large amounts are used over a long period of time.
health professionals can be found at:
www.biosyent.com
Consult your doctor immediately if any of these symptoms
This leaflet was prepared by:
HOW TO STORE IT
Pharmazeutische Fabrik Montavit Ges.m.b.H,
Salzbergstrasse 96, 6067 Absam, Austria
Remember to keep CATHEJELL Jelly 2% well out of the
reach of children when you are not using it.
Keep CATHEJELL Jelly 2% at room temperature. Do not
Distributed in Canada by: BioSyent Pharma Inc., Toronto,
keep CATHEJELL Jelly 2% in the bathroom medicine
Ontario, Canada M9W 5Z5
cabinet or other warm, moist places. Store in the original
Tel: 1-888-439-0013
Do not use CATHEJELL Jelly 2% after the expiry date
marked on the package.
REPORTING SUSPECTED SIDE EFFECTS
You can report any suspected adverse reactions associated with
the use of health products to the Canada Vigilance Program by
one of the following 3 ways:
--------------------------------------------------------------------------
•
Report online at
Call toll-free at 1-866-234-2345
Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to: Canada Vigilance Program
Health Canada
Postal Locator 0701E
Ottawa, Ontario
Postage paid labels, Canada Vigilance Reporting
Form and the adverse reaction reporting guidelines
are available on the MedEffect™ Canada Web site at
www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to the
management of side effects, contact your health professional. The
Canada Vigilance Program does not provide medical advice.
MORE INFORMATION
Important Note: This leaflet alerts you to some of the
times you should call your doctor. Other situations which
cannot be predicted may arise. Nothing about this leaflet
should stop you from calling your doctor with any
questions or concerns you have about using
CATHEJELL Jelly 2%.
NOTE: This CONSUMER INFORMATION leaflet provides
you with the most current information at the time of printing.
Source: http://www.cathejell.ca/downloads/CATHEJELLJellyProductMonographMay2014.pdf
15. Joint Advisory Committee on the Ethics of Investment 1 Role and Function of the Committee 1.2 SRI Reporting RequirementsIn July 2000 regulations came into force 1.1 Terms of Reference that obliges all pension funds to consider The Joint Advisory Committee on their policy, if any, on socially responsible the Ethics of Investment (JACEI) was
Septembre 2015 - N°9 Combien coûte la maladie d'Alzheimer ? Alain Bérard, Fondation Médéric Alzheimer, Directeur Adjoint Chloé Gervès, EA Management des Organisations de Santé - Ecole des Hautes Etudes en Santé Publique Equipe CAPRI - Institut Gustave Roussy Boursière de la Fondation Médéric Alzheimer - 2010 Roméo Fontaine, Laboratoire d'économie de Dijon (LEDi – UMR 6307 – Université de Bourgogne – CNRS – U1200 Inserm), Fondation Médéric Alzheimer







