
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Tm_ilm

-26 (For Individual Attention Only)
Table of contents
1 Introduction . 1
2 Tooth Discoloration . 2
2.1 Extrinsic Stain.3
2.2 Intrinsic Stain .4
2.3 Tooth Discoloration Due to Structural Abnormalities.5
2.4 Tooth Discoloration Due to Ageing .6
3 Techniques for Tooth Whitening. 7
3.1 Vital Tooth Bleaching .7
At-Home Whitening.7
In-Office Whitening .8
3.2 Non-Vital Tooth Bleaching .9
Walking Bleach Technique .9
Thermocatalytic Technique .9
4 Chemistry of Whitening . 10
5 An Open Legal Situation in the European Community? . 14
6 Illuminé Tooth Whitening System . 16
6.1 Indications and Contraindications .17
6.2 Interactions with Dental Materials .17
6.3 Tray Manufacturing.18
7 Illuminé home . 21
7.1 Product Description.21
7.2 Composition and Working Mechanism .21
7.3 Clinical Investigations.22
Clinical investigation of Illuminé home 10% by Swift et al at UNC (1997). 22
Clinical investigation of Illuminé home 10% by Barnes et al at the
University of Maryland (1998) . 24
Clinical investigation of Illuminé home 10% and 15% by Kihn et al at the
University of Maryland (2000) . 26
7.4 Step-by-Step Instructions.28
8 Illuminé office . 30
8.1 Product Description.30
8.2 Composition and Working Mechanism .31
8.3 In-Vitro Data Evaluation of the Enamel Surface Morphology in Teeth
Treated with Illuminé office by Sherman J, DENTSPLY Professional .32
Summary. 32
Methods . 32
Results. 33
Conclusion . 36
8.4 Clinical Investigations.36
A Clinical Study Evaluating the Efficacy of a New Chairside and Take-
Home Bleaching System – Final Report, January 27, 2000 by Bardwell D,
Papathanasiou A, Tufts University Dental Clinic . 37
Clinical Evaluation of a 15% In -Office Hydrogen Peroxide Tooth Whitening
Touch Up Agent - Updated Report April 24, 2000 by Kihn P W, Barnes D M, Adachi
E, The University of Maryland Dental School . 38
In-House Whitening Case Study FP 084 – Report February 4, 2000 by
Sherman J, DENTSPLY Preventive Care . 40
8.5 Step-by-Step Instructions.40
9 References and Selected Literature . 44
1 Introduction
A range of developmental abnormalities, acquired diseases, and degenerative changes result
in tooth discolourations which cannot be removed by simple brushing or professional tooth
cleaning and use of abrasives. For the treatment of such stains, chemical bleaching is a
conservative, tooth substance saving alternative to other restorative procedures such as
veneering or the placement of crowns.
For treatment of the above-mentioned diseases but also for pure cosmetic indications, the
DeTrey Division of DENTSPLY offers under the brand
a system of professional tooth
whitening products.
Illuminé home 10% and 15% are carbamide peroxide-based whitening gels for dentist-
monitored at-home treatment. The 15% fluoride-containing version offers a faster and more
intensive whitening effect than the conventional 10% version.
Illuminé office makes use of a new, innovative chemical concept and delivery form for the
controlled release of hydrogen peroxide. It lightens up to 9 shades in 30 minutes without heat,
light, or gingival isolation. Illuminé office is recommended as a pre-treatment boost to Illuminé
home Tooth Whitening Gels or as a stand-alone treatment.
The Illuminé Tooth Whitening products, their presentations forms, and the product information
(separate booklets for dentist, patient, and dental technician) have been specifically developed
to meet the requirements set by the European Commission for Medical Devices.
2 Tooth Discoloration
In the field of conservative aesthetic dentistry, tooth bleaching meets the demand of non-
destructive treatment of discoloured teeth in a variety of indications. However, success and
efficiency of the bleaching treatment, and the selection of the most suitable technique and
clinical procedure depend upon several issues to be considered. One characteristic all tooth
bleaching procedures have in common is the oxidative elimination or at least the decrease in
dental stains (please see Chapter 4 for details on the chemistry of this process). It is obvious
that bleaching success depends on the nature of the dental stain. To avoid masking of
pathological conditions, it is mandatory that the reason for the discoloration is understood prior
The clinically perceived shades of unstained teeth are determined by the inherent dentine and
enamel shades as illustrated in Figure 1. The overall shades of discoloured teeth are a
combination of tooth stain and the inherent tooth shade (Spouge 1973; Viscio et al. 2000).
Clinical (overall)
Spouge 1973; Viscio, Gaffar, Tu 2000
The clinically perceived shade of unstained teeth is determined by
the inherent dentine and enamel shades (Spouge 1973; Viscio et
al. 2000). P: pulp; D: dentine; E: enamel.
Commonly, tooth discoloration is classified regarding location and aetiology of the stain. In
general, dental discoloration can be grouped into extrinsic and intrinsic stain depending on the
location of chromophors causing stain. Extrinsic stain is confined to the tooth surface whereas
intrinsic stain, i.e. discoloration of the dental hard tissue, is caused by colouring compounds
deposited or permeated into dentine and/or enamel. On ageing, translucent enamel gets
thinner; the apparent tooth colour is then determined by the darker dentine shades. Structural
abnormalities of enamel or dentine count among other reasons for tooth discoloration.
2.1 Extrinsic Stain
Extrinsic stains are caused by the deposition of chromogenic material on the tooth surface
wherein adhesion of the chromogens on the tooth surface plays a critical role. The tenacity of
chromogen adhesion is determined by the interaction forces, but these mechanisms are not
yet fully understood. However, in the case of typical tannin chromogens causing e. g. tea,
coffee and red wine stains, adhesion of chromogens via saliva proteins on the tooth surface is
assumed as outlined in Figure 2 (Nathoo, 1997). Saliva proteins are selectively bonded via
calcium bridges, thus forming the pellicle. At the early stage of staining, chromogens
themselves are assumed to interact with the pellicle via hydrogen bridges. At this stage, food
stains can be removed by tooth brushing with standard tooth pastes. However, food and
tobacco stains are known to darken and become more tenacious over a longer period of time.
Especially the cervical portions of the teeth adjacent to the gingival margins often become
yellowish or brownish. Chemical analysis of aged stains of food and beverages revealed the
presence of furfurals and furfuraldehyde derivatives. These compounds are products of the
Millard reaction (nonenzymatic browning reaction), which is a series of chemical
rearrangements and reactions between sugars and amino acids (Viscio et al. 2000). Ageing of
extrinsic stains is not yet fully understood but seems to be related with the strengthening of the
bonding of chromophors to the tooth surface. Usually, these aged extrinsic stains cannot be
removed by tooth brushing. Professional cleaning with abrasive pastes or whitening with a
bleaching agent are necessary to lighten teeth. Tenacious surface stains are highly amenable
to bleaching, although stains are more difficult to remove from pits, fissures, grooves, or
enamel defects (Goldstein et al. 1995).
Tooth Discoloration: Chemistry
S.A. Nathoo. JADA 1997;128:6S-10S
Model of the interaction of tannins with the pellicle (Nathoo 1997).
Binding of colourless materials to teeth with subsequent reaction to chromogens may also
result in extrinsic stains. For example, colourless stannous fluoride is prone to reduction to tin
by sulfuridyl groups of pellicle proteins causing dark external metallic stain. Another example is
brown stain caused by redox reactions of chlorhexidine. Removing stains of antimicrobial
agents requires bleaching with oxygenating agents (Nathoo 1997).
2.2 Intrinsic Stain
Unlike extrinsic discoloration, intrinsic discoloration is due to chromogenic material located
within the dentine or enamel. Aetiology of intrinsic discoloration can be pre-eruptive and/or
post-eruptive. Intrinsic stains are not removable by brushing or any abrasive process, but can
be reduced by bleaching with agents penetrating enamel and dentine to decolourise the
chromogens. However, persistent intrinsic stains may be treated for a longer period than
extrinsic discoloration to lighten teeth and sometimes bleaching does not eliminate
discoloration totally.
The devastating effect of some medicamentation given systematically, especially during tooth
formation, is displayed by teeth of young people with yellow, brown or grey intrinsic stains of the
antibiotic tetracycline. The severity of stains and specific colour depends on the duration of
administration of tetracycline and the stage of tooth formation at the time of use. Teeth are
most susceptible to tetracycline discoloration in early childhood during tooth development and
even pre-natally, beginning with the second trimester of pregnancy. It is believed that
tetracycline is incorporated into the tooth structure during calcification via complexation with
calcium ions. The dye causing the discoloration results from a photochemical reaction of these
complexes when tetracycline affected teeth are exposed to sunlight, which is why the labial
surfaces of the incisors tend to darken more quickly to grey or brown while the molars remain
yellow for a longer time (Goldstein et al. 1995). Fully formed teeth in adults may be stained
from taking the tetracycline Minocycline for acne. This discoloration can be caused from
deposition of tetracycline in secondary dentine as well as from soaking in saliva (Haywood
Serious tetracycline stains are rather persistent and using bleaching, discoloration can often
only be reduced (Glockner et al. 1997; Haywood 1997). However, on extending treatment time
and concentration of active ingredient , lightening of even dark tetracycline stains can be
achieved (Leonard 2000, Haywood 2000).
Teeth can acquire intrinsic stains after eruption due to trauma on the teeth. Blood penetrates
into the dentine tubuli and the degradation products and iron complexes cause discoloration.
For the same reason, in some cases endodontically treated non-vital teeth exhibit discoloration
after some time. These discolorations can be reduced or even removed by bleaching.
However, with non-vital teeth, preference is usually given to internal bleaching, since in this
case it is more efficient than an external bleaching procedure (see Chapter 3).
Intrinsic stains caused by blood degradation products deposited within the dentine can also be
caused by haematological disorders such as erythroblastosis fetalis, thalassemia, and sickle-
cell anaemia because of the affected coagulation system (Nathoo 1997; Viscio et al. 2000).
Another source of intrinsic stains are aged dental materials like root filling materials, zinc oxide
eugenol cements, or amalgam (Glockner et al. 1997).
2.3 Tooth Discoloration Due to Structural Abnormalities
Fluorosis is a typical disease resulting in abnormal structure of the enamel. Endemic enamel
fluorosis is caused by excessive intake of fluoride during enamel formation and calcification,
resulting in discoloration and surface defects, so called "mottled enamel". Typically, affected
teeth show paper-white flecks, areas of yellow to brown or even dark grey stains. If mottling is
serious enough, enamel appears opaque, chalky without glaze and lustre of healthy teeth.
Bleaching is viewed to be useful to lower contrast between these white areas and the dark
stains to improve aesthetics. Bleaching can also be an adjunctive treatment preceding
veneering. However, if fluorosis caused severe lack of enamel or bare dentine, bleaching
should not be used at all (Goldstein et al. 1995).
2.4 Tooth Discoloration Due to Ageing
Changes in tooth colour almost inevitably accompany ageing. Most newly formed teeth exhibit
rather thick, even enamel which modifies the inherent base colour of the underlying dentine
resulting in an milky white appearance – the aesthetic ideal in today's society. Numerous
genetic, environmental, medical, and dental causes described above move away from this
ideal due to dental stains. As foods, beverages, and nicotine have cumulative staining effect,
teeth usually darken on ageing. Additionally, stains become more tenacious and darker due to
chemical reactions. Microcracks and defects in the enamel surface enable extrinsic
chromophors to penetrate into the enamel that results in intrinsic stain. On ageing, thinning of
enamel also causes a shift to a darker apparent colour. At the same time as the enamel
becomes thinner secondary dentine is deposited which is a natural protective mechanism. The
combination of all factors creates an old looking tooth. Usually, unless the enamel is badly
worn, bleaching is an efficient technique to lighten teeth of older patients (Goldstein et al.
3 Techniques for Tooth Whitening
Techniques for tooth whitening can be classified and described according to:
a) The vitality status of the teeth to be treated:
Vital bleaching vs. non-vital bleaching
b) Where and by whom the bleaching substance is applied:
In-office bleaching vs. at-home bleaching
3.1 Vital Tooth Bleaching
Vital teeth are bleached by application of the whitening substance on the external surface of
the teeth. The whitening procedure can be carried out in the dental surgery or at home by the
patient or by a combination of both techniques.
3.1.1 At-Home Whitening
[Synonyms: Nightguard vital bleaching]
At-home whitening involves the use of a 10 – 15% carbamide peroxide material applied in a
custom-made tray for a certain number of hours per day or during sleep. Treatment is carried
out by the patient himself, but the process is monitored by the dentist during recall
Advantages:
§ Bleaching is carried out with less aggressive chemicals.
§ The prolonged treatment time makes it easy to determine when the desired result is
§ Due to reduced chairtime, less costly for the patient.
§ Relatively long treatment time.
§ Not recommended in patients with limited compliance.
CRA data (Christensen, 1997) show that most products have lost 80% of their active
ingredient within 2 hours after placement in the mouth. All-night bleaching is therefore
questionable from the point of view of effectiveness. However, some patients prefer this
schedule as it does not interfere with their normal activities.
In most cases, the treatment period is between 1 and 6 weeks.
According to Haywood, 1997, the technique was first published in 1989 and can be traced
Guidelines by the American Dental Association, latest revision May 1998, recommend suitable
in vitro and clinical tests to investigate safety and efficacy.
3.1.2 In-Office Whitening
[Synonym: In-office bleaching]
With in-office bleaching, the whitening procedure is carried out in the dental surgery by the
dentist or under his supervision. The procedure usually takes 30 to 60 minutes. Several
appointments may be necessary to achieve the desired result.
At present, hydrogen peroxide is considered to be the most effective vital bleaching agent for
in-office application. According to Haywood, 1998, this technique was already applied by
Harlan back in 1884.
Bleaching agents vary from a solution of 35% hydrogen peroxide to various gels of greater or
lesser content. The process can be applied to an entire arch or to a single tooth only.
Nowadays, it is believed that an external energy source (heat, light, laser) does not enhance
the whitening reaction.
Advantages:
§ Instant result: Patient leaves surgery with whiter teeth.
§ The complete process is under the control of the dentist.
§ Process is independent from patient compliance.
§ Gingival isolation by rubberdam or a rubberdam substitute is necessary.
§ Complex application technique and use of potentially harmful, highly aggressive chemicals.
§ Higher costs incurred for the patient due to extended chairtime.
§ Several appointments may be necessary to achieve the desired result.
With Illuminé office (see Chapter 8.1), most disadvantages of conventional in-office systems
3.2 Non-Vital Tooth Bleaching
[Synonyms: Internal bleaching]
Non-vital tooth bleaching refers to the whitening of tooth discolorations caused by traumatic
damages of the pulp, pulp necrosis, or endodontic treatment.
Non-vital teeth are bleached by application of the whitening substance into the prepared pulp
chamber and coronal part of the root canal. In addition to this internal bleaching, a suitable
whitening substance may also be applied onto the external surfaces of the tooth.
30% hydrogen peroxide solution alone or mixed with sodium perborate, or sodium perborate
mixed with water are common formulations for non-vital tooth bleaching. The whitening agent is
supplied into the pulp chamber in 2 different techniques:
3.2.1 Walking Bleach Technique
The bleaching agent (sodium perborate mixture) is sealed in the pulp chamber to allow slow
activation over several days.
3.2.2 Thermocatalytic Technique
The complete treatment is carried out in the surgery and involves the repeated application of a
30% hydrogen peroxide solution which is activated by heat over a period of about 30 minutes.
4 Chemistry of Whitening
Bleaching is a chemical process which is widely applied for whitening materials mostly using
oxidising compounds, e.g. hydrogen peroxide, chlorine or sodium hypochlorit. Although
bleaching processes are complex sequences of chemical reactions, the underlying principle of
the vast majority is the stepwise oxidation of dyes to decolourise them. Total oxidation of
organic chromogens via several intermediates ends up in the final products carbon dioxide
CO2 and water H2O. The extent and the rate of oxidation can be controlled by the bleaching
conditions (e.g. oxygenating agent, concentration, duration of bleaching, and temperature).
Control of chemical bleaching is important for its feasibility in dentistry with respect to dental
health and safety.
In dentistry, modern bleaching materials contain oxidising peroxide compounds, i. e. hydrogen
peroxide H2O2 or carbamide peroxide (CP). Carbamide peroxide is a 1:1 complex of urea and
hydrogen peroxide. Hydrogen peroxide is stabilised in this complex. In the presence of
compounds prone to oxidation, hydrogen peroxide is released (Figure 3). The released
hydrogen peroxide, but not urea has an oxidising capacity. 1.00 g carbamide peroxide is
equivalent to 0.36 g hydrogen peroxide when completely released.
Chemistry of Bleaching
Reactive oxidizing radical
Carbamide Peroxide = Stabilized Hydrogen Peroxide
Carbamide peroxide (CP) is a 1:1 complex of urea and hydrogen
peroxide. Hydrogen peroxide is stabilised in this complex. In the
presence of compounds prone to oxidation, hydrogen peroxide is
The mechanism of vital tooth whitening with peroxide containing bleaching gels is illustrated
schematically in Figure 4. The bleaching gel is administered on the surface of discoloured
teeth. Peroxide is released from the gel and can permeate into the enamel and dentine.
Therefore, not only extrinsic staining dyes but also intrinsic chromogens can be oxidised to
colourless products.
Illuminé : Tooth Whitening System
Dentin Enamel Pellicle
Discoloration caused by
Penetration of peroxide
Decolorized dentin and
which oxidize the
Schematic illustration of the mechanism of bleaching with
peroxides. Peroxide is released from the bleaching gel
administered on the tooth surface and diffused into tooth hard
tissues. Peroxide attacks extrinsic and intrinsic staining
chromogens which results in decolourising dyes via an oxidation
The oxidation process of organic compounds with hydrogen peroxide is a complex series of
reactions. Hydrogen peroxide is a metastabile liquid with a rather high tendency to
decomposition into water and oxygen according to
The decay of hydrogen peroxide is enhanced with increasing temperature and by irradiation
with UV light, and also depends on the pH value. To reduce breakdown and to extend shelf life,
hydrogen peroxide is usually available in acidic aqueous solutions and has to be stored dark
and refrigerated. A 30% aqueous solution of pure hydrogen peroxide has roughly pH 3. An
elevated pH accelerates decomposition. In the presence of catalysts, like several metal ions
and metal oxides, and natural enzymes, e.g. peroxidase and catalase, decomposition is
strongly enhanced. The hydrogen peroxide breakdown is a radical process. The first step of
the radical mechanism is the formation of the hydroxyl radicals HO· inducing a chain reaction.
In the course of the reaction dioxygenyl radicals HOO· are also formed. Hydroxyl and
dioxygenyl radicals are highly reactive and attack organic material to oxidise it. Especially
compounds with unsaturated double bonds are highly prone to oxidation with these radicals.
Typically, staining organic chromogens are characterised by conjugated double bonds in the
molecule, which is the structural reason for the colour. Via several intermediate steps, the
oxidation reaction of the dye molecules results in colourless hydrophilic molecules comprising
hydroxyl groups (HO-groups). This is illustrated schematically in Figure 5.
Completely bleached
No further whitening
Schematic illustration of the chemical oxidation reaction of dye
molecules comprising unsaturated double bonds with hydrogen
peroxide to colourless molecules. After conversion of all stains to
colourless product the saturation point is reached. Further
bleaching would only cause degradation to carbon dioxide and
water but would not increase the whitening effect (overbleaching).
As bleaching proceeds, teeth continually lighten. When all chromophors are converted to
colourless molecules, the so-called saturation point is reached. Further bleaching, i.e.
overbleaching, would cause further oxidation resulting in degradation to carbon dioxide and
water, but would not increase the whitening effect. Neither does it improve the brightness of the
teeth. At the saturation point, further lightening of teeth slows down dramatically. Excessive
overbleaching bears the risk to oxidise proteins of the enamel and dentine which may cause
significant alteration of the enamel and dentine structure. Reduction of the tooth structure by
loss of enamel may be the consequence (Goldstein et al. 1995). Therefore, it is demanded
that in-office bleaching be carried out by a dentist and that home-bleaching be supervised and
regularly controlled by a dentist. Overbleaching with over-the-counter nightguard products and
without supervision by a dentist is of particular concern (Haymann et al. 1997).
5 An Open Legal Situation in the European Community?
When tooth whitening products started to appear in Europe at the beginning of the nineties, a
dispute has arisen whether these products are legally defined as cosmetics or as medical
Tooth Whiteners as Cosmetic Products
On 15th February 1996, Mrs. Bonino announced in the name of the Commission of the
European Community that all tooth whitening products are cosmetic products as defined by the
Council Directive 76/768/EEC. The active substance of tooth whitening products, hydrogen
peroxide, is regulated by the Directive 92/86/EEC. The 15th adaptation of this Directive of 1st
July 1993, allows a maximum concentration of 0.1% hydrogen peroxide. As the tooth whitening
products commonly used contain or release at least 3% hydrogen peroxide, Mrs. Bonino
concluded that the use of such products by dentists and the public was not permitted.
The Scientific Committee on Cosmetic Products and Non-food Products intended for
Consumers (SCCNFP) reviewed the safety of hydrogen peroxide in tooth-whitening products
following the request to increase the permitted level in Annex 3 of Directive 76/768/EEC from
0.1 to 3.6%. In the plenary meeting of 17th February 1999, the SCCNFP adopted the opinion
that it would be inappropriate to provide products with more than 0.1% hydrogen peroxide as
cosmetic products. This decision was due to the fact that the contra-indications and warnings
necessary for products with a higher concentration of hydrogen peroxide were found
incompatible with the nature of a cosmetic product.
Therefore, at present tooth-whiteners containing or releasing more than 0.1% hydrogen
peroxide cannot be marketed as cosmetic products within the member states of the EC.
Tooth Whiteners as Medical Devices
The statement by Mrs. Bonino that all tooth-whitening products are cosmetics can be
challenged easily. According to Article 1 of the Council Directive 93/42/EEC of 14th June 1993,
materials intended by the manufacturer to be used for the purpose of treatment or alleviation of
disease and which do not achieve their principle intended actions by pharmaceutical,
immunological or metabolic means are medical devices. Therefore, tooth-whitening products
intended by the manufacturer to be used for tooth discolourations caused by disease such as
pulp necrosis or by tetracycline medicamentation are medical devices.
This opinion is neither shared by the Notified Bodies (institutions certifying compliance of
manufacturers with EC regulations regarding a quality management system for the
development and the production of medical devices) nor by the local Health Authorities which
have to adopt Mrs. Bonino's view. This may result in further court actions similar to those
already ongoing:
In the UK in 1998, the High Court ruled that a tooth-whitener product is a medical device and
that the Department of Trade and Industry (DTI) was wrong in ban its use. The DTI appealed
and on 1st July 1999, the Court of Appeal overturned the previous High Court ruling. Leave is
now being thought from the House of Lords to appeal against the later ruling. The ruling is
expected for fall/winter 2001.
In Germany, the Verwaltungsgericht Düsseldorf (16 K 6063/99) ruled that a tooth whitener is
considered a medical device. The Competent Authority (Bezirksregierung Düsseldorf)
appealed and a decision is expected for fall 2001. Both parties of this dispute indicated to
appeal if not successful.
As long as the European Commission do not reconsider their unfortunate and wrong statement
of 1996, the dispute will go on until the European Court will come to a ruling.
6 Illuminé Tooth Whitening System
The Illuminé™ Tooth Whitening System is a complete concept for external bleaching that
covers both in-office and at-home bleaching with different peroxide concentrations (Figure 6)
to gain best results according indications, safety, and efficiency.
carbamide peroxide (cp)
office treatment
Overview of the Illuminé™ Tooth Whitening System.
Illuminé home Tooth Whitening Gels may be used alone or following treatment with an in-office
Illuminé office In-Office Tooth Whitener is recommended as a pre-treatment boost to Illuminé
home Tooth Whitening Gels or as a stand-alone treatment completed in 1 to 3 office visits. If
used as a boost to Illuminé home, the take-home regime may be abbreviated to a 3-day
The Illuminé Tooth Whitening products, their presentation forms, and the product information
(separate booklets for dentist, patient, and dental technician) have been specifically developed
to meet the requirements set by the European Commission for Medical Devices.
6.1 Indications and Contraindications
Indications
1. Tooth discolouration caused by:
§ Staining from foods, drinks, and tobacco with penetration into the tooth substance
§ Age-dependent degenerative changes
§ Tetracycline (first and second grades) or minocycline medicamentation
§ Fluorosis, especially brown pigmentation
§ Pulp necrosis and/or endodontic treatment
§ Masking of enamel mottling
§ Genetically determined dark teeth
§ Whitening of discoloured teeth prior to restorative and/or prosthodontic measures, e. g.
The best results are with patients whose teeth are discoloured to yellow, orange, or light brown.
§ Use during pregnancy or lactation.
§ Known allergy to hydrogen peroxide or other ingredients of the products.
§ Extrinsic stains which can easily be removed by professional tooth cleaning and which can
be prevented by thorough oral hygiene.
§ Use in heavy smokers unless they refrain from smoking while wearing the tray.
6.2 Interactions with Dental Materials
Existing tooth coloured restorations may not match the lighter shade of teeth after the
treatment and may need to be replaced.
The Illuminé products should be used before the placement of composites, veneers or crowns,
in order to maintain a close match of tooth colour. We also recommend that the patient wait a
minimum of two weeks after the treatment for the tooth discolouration to stabilise before
performing anterior restorations.
Some old restorations (e.g. amalgams) may leave dark stains in the tray. This is normal and
6.3 Tray Manufacturing
A well fitting tray with a proper gingival seal and margins just away from the gingiva is essential
to reduce irritation of the gingiva during the whitening procedure to a minimum.
For tray fabrication, information on which teeth are planned to be treated as well as the
whitening technique (home treatment only or office treatment) should be available. For
communication between clinician and technician, a form (Figure 7) is supplied on the last page
of the "Instructions for tray fabrication" booklet.
The tray for Illuminé office (the gel reservoir of which is larger than that of the tray designed for
sole home application) may also be used for short-term subsequent treatment with Illuminé
Generate Stone
Pour the disinfected, rinsed impression with dental stone.
Trim the stone cast so that the base is parallel to the occlusal plane of the posterior teeth and
that the base extends 2-4 mm past the gingival border.
Tray Fabrication
For tray fabrication, please also see illustration (Figure 7).
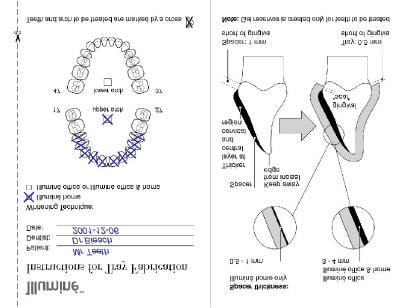
Creating the Reservoirs
1. Once the stone model has dried to an effective hardness apply an acrylic spacer (e. g.
Triad1 Gel) to the teeth on the model, thus creating reservoirs in the gel tray.
2. Apply the spacer only to teeth of aesthetic value.
3. Stay approximately 1 mm from the gingival margin when applying the spacer.
4. Do not cover any interproximal areas.
5. Do not apply spacer to occlusal surfaces or incisal edges.
6. Apply a spacer to the facial surfaces of the teeth to be whitened.
For treatment with Illuminé home tooth whitening gels alone, the thickness of the spacer is
For treatment with Illuminé office or a combination treatment with Illumine office and home,
the thickness of the spacer is 3-4 mm.
Note: If the tray will be used for an in-office procedure alone or an in-office procedure in
combination with short-time treatment with Illuminé home tooth whitening gels, the thickness
of the spacer is increased according to the directions for use of the respective product.
Wait until spacer has set or light-cure spacer, respectively.
7. Wipe the cured spacer surfaces with a 7. gauze dampened with alcohol to remove the
slightly tacky surface that remains on the cured acrylic spacer.
1 DENTSPLY DeTrey, Dreieich (D).
Forming the Tray
1. Use a medium thick heat/vacuum sheet2 for tray fabrication.
2. Place the vacuum sheet (pebble finish towards the stone model) on a heat/vacuum tray-
forming machine.
3. Soften the vacuum sheet until it sags 2 to 5 cm or as directed by the Manufacturer.
4. Engage the vacuum, and slowly lower the heated gel tray onto the stone model to avoid
generating wrinkles and folds.
5. Allow the sheet ample time under vacuum for good retention and definition.
6. Allow the sheet to cool prior to trimming.
Trimming the Tray
1. Remove the gel tray from the stone model.
2. Using scissors trim the tray approximately 2-3 mm beyond the teeth.
3. Trim and refine the remaining portion with smaller, precision scissors (Scallop around the
interdental areas).
4. Trim the tray approximately 1/4 - ½ mm short of the gingival margin.
5. Place the trimmed tray back onto the stone model to verify extensions.
6. Finish margins with rotary instruments or slightly flame polish tray edges.
7. Wash and disinfect the whitening tray. Dry thoroughly.
8. Place in storage case or other suitable container and deliver to dentist.
2 Erkodur (clear), 0.8 mm thickness, Reorder # 521108 (Erkodent®, D-Pfalzgrafenweiler)
7 Illuminé home
7.1 Product Description
Illuminé home Tooth Whitening Gels may be used alone or following treatment with an in-office
tooth whitener. When used as following treatment the same tray of the prior Illuminé office
treatment can be used.
Illuminé home Tooth Whitening Gels contain the active ingredient of carbamide peroxide, which
has shown to be most effective for the treatment of tooth discolourations at home.
Illuminé home 10% Tooth Whitening Gel contains 10% carbamide peroxide, releasing H2O2 in
a concentration of about 3.6%.
Illuminé home 15% Tooth Whitening Gel contains 15% carbamide peroxide, releasing H2O2 in
a concentration of about 5.4%, and in addition sodium fluoride.
7.2 Composition and Working Mechanism
Illuminé home 10% and 15% are colourless tooth whitening gels for nightguard or day-time
home treatment comprising carbamide peroxide as active bleaching ingredient. The
composition is shown in Figure 8.
Illuminé™: Tooth Whitening System
Illuminé™ home 10% & 15%
• Nightguard/day-time home bleaching gel• 10% / 15% carbamide peroxide equivalent 3.6% / 5.4% H O
• Gelating agent Carbomer (modified polyacrylic acid)• Sodium hydroxide to buffer pH to 5.7-6.5• Illuminé home 15% comprises 0.22% sodium fluoride• Mint flavor• Solvent glycerine
Composition of Illuminé home 10% and 15%.
The gelating agent used within both formulations is Carbomer which is modified polyacrylic
acid. The pH of this polyacid is buffered to 5.7 – 6.5 with sodium hydroxide. The formulation
provides good adherence of the gel on the tooth surface. Peroxide is released almost
completely after two hours (Christensen, 2001).
Additionally, the 15% formulation comprises sodium fluoride to reduce sensitivity.
7.3 Clinical Investigations
Safety and performance of the two carbamide peroxide based tooth whitening gels for dentist-
monitored home application, marketed by DENTSPLY DeTrey under the brand Illuminé home,
were investigated in 3 separate formal investigations.
7.3.1 Clinical investigation of Illuminé home 10% by Swift et al at UNC (1997)
The material used in the investigation is identical to the formulation of Illuminé home 10%.
Design, method, and material of the investigation are summarised in Figure 9, the findings are
summarised in Figure 10 and Figure 11.
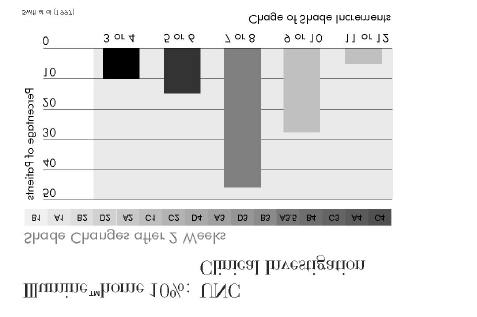
The investigators conclude that the test material was an effective agent for lightening of
discoloured teeth.
Illumine™home 10%: UNC Clinical Investigation (1997)
Double-blind controlled investigation
Number of patients 29 patientsControl materials
50% of patients treated with test material,50% of patients treated with placebo gel for 14 days,then switch of region and further treatment for 14 daysIndividual traysThickness of reservoir: 0.5mmShade determination with Vita Lumin guide, pre-treatment, 2 weeks, 4 weeks, 3 + 6 mths post-treatment
Figure 10
Illumine™home 10%:UNC Clinical Investigation (1997)
• Shade changes in placebo group occurred in 35% of the cases.
• Whitening remained efficacious after 3 months in 97% of the cases and
after 6 months in 90% of the cases.
• Transient tooth sensitivity was 17% in placebo group and 7% with
Illuminé home 10%.
ð Effective agent for lightening discoloured teeth
Figure 11
7.3.2 Clinical investigation of Illuminé home 10% by Barnes et al at the University of
Maryland (1998)
The material used in the investigation is identical to the formulation of Illuminé home 10%.
Design, method, and material of the investigation are summarised in Figure 12, the findings
are summarised in Figure 13 and Figure 14.
The investigators concluded that the test material when administered under the supervision of
a dentist is an effective whitening agent.
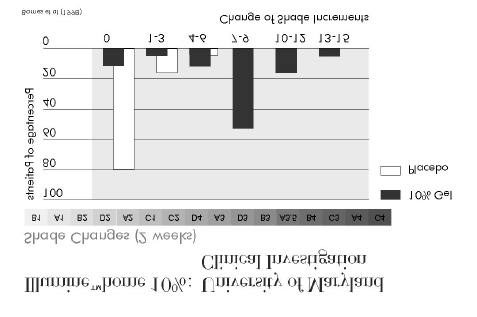
Illumine™home 10% :
University of Maryland Clinical Investigation (1998)
Double-blind clinical trial
Number of patients 61 patientsControl materials
50% of patients treated with test material,50% of patients treated with placebo gel for 14 daysIndividual traysThickness of reservoir: 0.5-1mmShade determination with Vita Lumin guide, pre-treatment, 14 days, 3 and 6 months post-treatment
Figure 12
Figure 13
Illumine™home 10% :
University of Maryland Clinical Investigation (1998)
• Whitening remained efficacious at 3 months in at least 76% of the cases
and at 6 months in at least 64% of the cases.
• Transient tooth sensitivity and/or gingival sensitivity occurred in approx.
2/3 of the patients.
• No significant changes in gingival condition.
ð Effective and safe tooth whitening system
Figure 14
7.3.3 Clinical investigation of Illuminé home 10% and 15% by Kihn et al at the
University of Maryland (2000)
The whitening gels investigated in this clinical trial are identical to the formulations of Illuminé
Design, materials, and methods used in the investigation are summarised in Figure 15, the
outcome is summarised in Figure 16 and Figure 17.
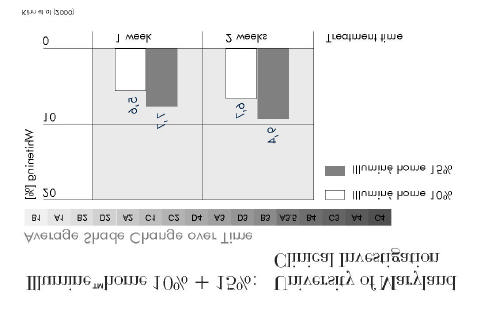
Illumine™home 10% + 15%:
University of Maryland Clinical Investigation (2000)
Double-blind clinical trial
Number of patients 57 patientsMethod
50% of patients treated with 15% gel,50% of patients treated with 10% gelIndividual traysThickness of reservoir: 0.5-1mmTreatment time: 14 days (≥ 4h/d)Shade determination with Vita Lumin guide, pre-treatment, 1 week, 2 weeks after begin of treatment,2 weeks post-treatment
Figure 15
Figure 16
Illumine™home 10% + 15%:
University of Maryland Clinical Investigation (2000)
• At one week, no significant difference in shade change with 10% and
• With the 15% gel, a significantly more intense whitening was achieved
after 2 weeks of treatment and 14 days after end of treatment.
• No significant difference in level of tooth sensitivity between the two
ð Both gels offer an effective whitening treatment.
The 15% gel offers a more intensive colour change.
Figure 17
7.4 Step-by-Step Instructions
Prior to use of the Illuminé products, it is mandatory to read the respective Directions for Use. Here you will also find the complete instructions for use, information on precautions which have to be taken prior to treatment and warnings to be considered.
Brush and floss teeth prior to wearing the tray.
Prior to first use of syringe,
Fix applicator provided on
Open applicator by breaking off tip (retain tip to close applicator after use of syringe).
Place small amounts of gel
Close applicator on syringe.
towars facial side of teeth to be treated.
Wear the tray throughout the night or as directed (a minimum of 2 hours in mouth is suggested).
Insert gel tray in the mouth
Wipe excess material from the
gums or tissues with finger or tooth brush.
Do not eat or drink anything or smoke while wearing the tray.
After treatment, remove tray.
Rinse your teeth. Brush away any remaining gel.
Clean the tray with warm
Dry tray thoroughly and store
water and a tooth brush.
8 Illuminé office
8.1 Product Description
Illuminé office is unique in both technique and formulation and makes use of a new chemical
concept for the controlled release of hydrogen peroxide.
Illuminé office is a 15% hydrogen peroxide-based treatment which will provide with significantly
noticeable whitening results in 30 minutes. The process requires no light or heat activation.
Illuminé office is recommended as a pre-treatment "boost" to Illuminé home Tooth Whitening
Gel, or as a stand-alone treatment, completed in 1 to 3 office visits. If used as a boost to
Illuminé home, the take-home regimen may be abbreviated to a 3-day treatment.
Each kit contains two (2) syringe sets and an applicator tip for each. Each syringe set
comprises one syringe containing a 30% hydrogen peroxide solution (syringe A) and one
powder syringe (syringe B) with an attached stopcock. Each syringe set contains enough
material to whiten 1 arch.
The hydrogen peroxide solution and the gelating powder are separated in two syringes to
maintain stability during storage. The contents of the syringes A (hydrogen peroxide) and B
(powder) are mixed according to the step-by-step instructions directly before administration.
The mixed 15% hydrogen peroxide bleaching gel is applied in the custom-fitted tray. After
setting of the gel to a rubbery semi-solid (setting time: 30 to 60 seconds) the tray is seated on
the teeth. Due to the semi-solid consistency and the good adherence on the tooth surface
migration of the material can be avoided. Therefore, gingival isolation should not be required if
the instructions are followed, and a well-fitting bleaching tray has been prepared with adequate
(3 – 4 mm depth) reservoirs for each tooth to be bleached.
8.2 Composition and Working Mechanism
Syringe A contains: 30% hydrogen peroxide
Syringe B contains: Poly (Methyl Vinyl Ether/Maleic Anhydride) mixed calcium/sodium salts,
titanium dioxide
Figure 18
Unique performance of the Illuminé office bleaching system.
Illuminé office has an unique rubbery semi-solid consistency which allows safe administration
without gingival isolation, and improves comfort and economy of in-office bleaching. The
advantageous consistency is achieved with mixed sodium/calcium salts of poly(methyl vinyl
ether/maleic anhydride) which is a copolymer (PVM/MA-copolymer) and exhibits gelating
properties. This copolymer is part of the powder in syringe B. When mixed with the hydrogen
peroxide solution that contains 70% water, the copolymer absorbs the water and swells.
Setting to the semi-solid is due to the hydration reaction. It is not possible to combine the
powder with the hydrogen peroxide ahead of time as the mixed material will form a skin and can
no longer be extruded from the syringe. The mixture progressively releases the peroxide over
time. Therefore, the products must be mixed at the point of use.
Additionally, the PVM/MA-copolymer buffers the pH of the hydrogen peroxide solution (pH 3) to
be between 5.6 and 5.9 in the mixed material. An enamel surface study was conducted by
scanning electron microscopy which indicates that exposure to the final mixed product for up to
90 minutes will NOT demineralise the enamel and effect the tooth surface structure (see In-
vitro data, Chapter 8.3).
The PVM/MA-copolymer provides the good adherence of the rubbery semi-solid to the tooth
surface which is necessary for optimal release of the hydrogen peroxide to the tooth and best
bleaching efficiency.
Titanium dioxide (TiO2) causes the opaque white colour of the mixed gel which makes optical
control of proper seating of the gel on the teeth more comfortable.
8.3 In-Vitro Data
Evaluation of the Enamel Surface Morphology in Teeth Treated
with Illuminé office by Sherman J, DENTSPLY Professional
8.3.1 Summary
The study was conducted to determine if the Illuminé office In-Office Tooth Whitener has any
effect on tooth surface morphology when placed directly on the tooth surface for designated
periods of time. Mixed product was placed directly on the surface of extracted human teeth for
30 minutes, 60 minutes, 90 minutes, or a series of three 30-minute treatments. Scanning
electron microscopy was used to evaluated surface morphology changes post-treatment
compared to untreated tooth surface. The results of the study suggest that Illuminé office does
not produce significant morphological changes in the surface of a tooth when administered for
up to 90 minutes or when administered for a total of 90 minutes through three individual 30-
minute treatments in an in vitro environment.
8.3.2 Methods
Application of Illuminé office
Extracted human incisors were treated on the longitudinal half of the anterior surface with the
mixed bleaching gel. The material was placed directly on the tooth surface and allowed to set
up. A dab of Triad Gel was placed on the posterior of each tooth and light-cured to designate
the treated side of the tooth. The remaining half of each tooth served as an untreated control
for the tooth. The teeth were partially and intermittently submersed in deionised water and
maintained at 37°C for the duration of the treatment. Treatment times were 30 minutes,
60 minutes, 90 minutes or a series of three 30-minute treatments at 24 hour intervals. The
material was gently removed with gauze and the teeth were placed in labelled containers of
deionised water.
Positive control
Etching gel was applied to the surface of one tooth for two minutes. The tooth was then placed
in a labelled container of deionised water.
Evaluation
Upon completion of treatment, the teeth were sent to DENTSPLY Caulk for surface
morphology examination by scanning electron microscopy (SEM). Treated and untreated
areas of each tooth were visually examined and photographed at magnification ratios of
2500:1 and 5000:1.
8.3.3 Results
The SEM photographs suggest that Illuminé office does not produce significant morphological
changes in the surface of a tooth when administered for up to 90 minutes or when
administered for a total of 90 minutes through three individual 30-minute treatments in an in
vitro environment (Figure 19 - Figure 22). In difference to the treatment with Illuminé office,
after etching teeth of the positive control group exhibit typical etching pattern of the enamel
surface (Figure 23).
Figure 19
Scanning electron microscopy photographs of untreated teeth and
of teeth treated with Illuminé office for 30 min.
Figure 20
Scanning electron microscopy photographs of untreated teeth and
of teeth treated with Illuminé office for 60 min.
Figure 21
Scanning electron microscopy photographs of untreated teeth and
of teeth treated with Illuminé office for 90 min.
1 Intervall of 24 h
Figure 22
Scanning electron microscopy photographs of untreated teeth and
of teeth treated with Illuminé office for 3 x 30 min with an interval of
Figure 23
Scanning electron microscopy photographs of untreated teeth and
positive control after etching for 2 min.
8.3.4 Conclusion
Under in vitro conditions the morphology of the enamel surface is not significantly altered when
treated with Illuminé office up to 90 min.
8.4 Clinical Investigations
Safety and performance of the professional in-office tooth-whitening product marketed by
DENTSPLY DeTrey under the brand Illuminé office was investigated in two separate formal
clinical investigations and one internal user evaluation. When used as a stand-alone treatment
and for an application time of 30 minutes, the average whitening effect achieved in the
investigations referred to was 3.4 vs. 5.8 vs. 7.2 shades (measured at the Vita Lumin shade
8.4.1 A Clinical Study Evaluating the Efficacy of a New Chairside and Take-Home
Bleaching System – Final Report, January 27, 2000 by Bardwell D, Papathanasiou A,
Tufts University Dental Clinic
Objectives: To evaluate the effectiveness and safety of a 15% hydrogen peroxide in-office
whitening product followed up by home application of a 10% carbamide peroxide whitening gel.
Materials and Method: The upper anterior teeth of 24 patients with a shade no lighter than
A3 were selected. Shades were assessed using a Vita Lumin Shade Guide. The in-office
whitening product (used for 30, 45, and 60 minutes) and the home whitening gel were applied in
custom-made trays. Shade recording was done prior to application, immediately after in-office
treatment, 24 hours after in-office treatment and 24 hours, 72 hours, and 7 days after home
Results: Whitening could be attained quickly with the in-office system and was enhanced by
subsequent home application of the 10% carbamide gel.
The final result was slightly different for the 30-minute, 45 and 60-minute in-office treatment
Illumine™office: Tufts University Dental Clinic
Clinical Investigation (2000)
Average shade changes
Change in Shade Increments
office application office application office application
50% of the patients treated experienced tooth sensitivity which improved fast and did not return
for any patients at completion of the investigation.
8.4.2 Clinical Evaluation of a 15% In -Office Hydrogen Peroxide Tooth Whitening
Touch Up Agent - Updated Report April 24, 2000 by Kihn P W, Barnes D M, Adachi E,
The University of Maryland Dental School
Objectives: To investigate the performance when used as a "touch up" treatment for
previously whitened teeth
Materials and Methods: 31 patients with previously whitened teeth were treated with Illuminé
office applied in a custom-made tray (where available, the existing whitening trays were used).
For 10 patients, the application time was 30 minutes, for 11 patients it was 45 minutes, and for
another 10 patients it was 60 minutes. Shade determination was carried out with a Vita Lumin
shade guide and was documented by colour transparencies at 1:1 magnification:
Pre-application,
Immediately post-application,
72 hrs post-application.
Results: Of the 31 patients who began the study, 2 did not return for the 72-hour recall and
were excluded from the analysis as were 2 patients presenting with shades lighter than B1.
Frequency distribution for shade change of the remaining 27 patients is given in Table 2.
Illumine™office: University of Maryland
Clinical evaluation (2000)
Shade changes 72 hours after treatment
Percentage of Patients
Change in shade increments
The average shade changes are given in Table 3. The shade change for the 30-minute
treatment was 3.4 at 72 hours post-treatment.
Average shade changes
Immediate post-op
72-hrs post-op
Tooth sensitivity occurred in the majority of all cases (Table 4), but there was only 1 case with
lingering tooth sensitivity at the 72-hour recall.
Side effects: Tooth sensitivity
During treatment
Day of treatment
Day after treatment
2 days after treatment
3 days after treatment
7 patients experienced mild gingival burns, i. e. a whitening of the tissue, which left behind an
erythematous area from contact with the bleaching material. In 6 of these cases, the problem
was associated with an ill-manufactured tray.
6 of the patients reported a burning sensation of the gingiva during treatment which
disappeared after tray removal.
§ The test material proved to be an efficient agent for rewhitening.
§ Proper tray fabrication is important for the prevention of gingival burns.
§ Side effects were mild and manageable by both the clinician and the patient.
8.4.3 In-House Whitening Case Study FP 084 – Report February 4, 2000 by Sherman
J, DENTSPLY Preventive Care
Objectives: To evaluate the effectiveness of Illuminé office for whitening teeth when applied
for 15 or 30 minutes
Materials and Methods: 12 subjects were divided in 2 treatment groups (1 for 15-minute,
1 for 30-minute application times). Shade evaluation with Vita Lumin Shade Guide was carried
out prior to application, immediately after application, and 24 hours after application. The in-
office whitening product was applied in custom-made trays.
Results: Both the 15-minute and the 30-minute treatments were effective to whiten teeth.
However, the 30-minute result proved to be superior (Table 5).
Illuminé office: Clinical in-house investigation (DENTSPLY Preventive
Care)
Average change from baseline to 24 h recall
Treatment time
30 minutes
15 minutes
No patient complained of sensitivity during treatment. One patient reported significant post-
treatment sensitivity, but by the 24-hour recall the sensitivity was gone. 5 patients experienced
isolated minor gingival irritation that had resolved by the 24-hour recall.
Conclusions: Significant tooth whitening can be achieved with Illuminé office as a stand-
alone treatment.
8.5 Step-by-Step Instructions
Initial Consultation
1. Review the patient's medical and dental history.
2. Perform an intraoral examination of the, soft tissue, periodontal, and teeth health.
3. Inform the patient that depending on the nature of the stain, an extended treatment may be
required to attain the desired effect.
4. A full prophylaxis (using Nupro Prophy Paste) and fluoride treatment is recommended
before starting the bleaching process.
5. The complete procedure should be discussed with the patient, reviewing any potential
problems and side effects.
6. Inform the patient that any existing tooth coloured restorations may not match the lighter
shade of teeth after the treatment and may need to be replaced.
7. Use a suitable shade guide, e. g. Biodent3 or Vita4 Lumin Vacuum shade guide, to make a
shade determination at baseline. Enter the determined shade into the patient's record.
Take Impression
Make an alginate or elastomer impression of the arch to be treated (It is highly recommended
that the teeth be treated one arch at a time, so that the patient can see the changes.)
Tray Fabrication and Communication with Dental Laboratory
Gingival Isolation
Gingival isolation should not be required if the instructions are followed, and:
§ a well-fitting bleaching tray has been prepared with adequate (2-4 mm depth) reservoirs for
each tooth to be bleached
§ the Illuminé material has been allowed to properly set, assuring that it will adhere to the
tooth surface and not migrate, and
3 DENTSPLY DeTrey
4 Vita is a registered trademark of Vita Zahnfabrik.
§ care has been taken during placement in the mouth to assure that the material has not
contacted the gingiva.
If, however, the instructions cannot be followed, or if edentulous or interdental gingival spaces
such as diastemas are present, we strongly recommend that the gingival tissue in question be
protected via a petrolatum jelly such as Vaseline®, an isolation resin (paint-on rubber dam), or
a conventional rubber dam.
Step-by-Step Instructions for Application of Illuminé office
Prior to use of the Illuminé products, it is mandatory to read the respective Directions for Use. Here you will also find the complete instructions for use, information on precautions which have to be taken prior to treatment and warnings to be considered.
Always hold syringe A
Turn valve to "on" position.
upright, remove cap, and screw syringe B onto syringe A.
Mix from syringe A to
Turn valve to "off" position.
syringe B 3-5 times.
Mixed material must end in syringe A.
Remove syringe B from the
Connect applicator tip to
upright syringe A.
upright syringe A.
Extrude a small amount of
material into each reservoir.
Material must become semi-viscous.
Upon required viscosity,
Remove excess material
seat the tray and press
Let patient wait for 30
Remove tray and clean
teeth thoroughly.
9 References and Selected Literature
1. American Dental Association (1998). Home-use tooth whitening products. Acceptance
Program Guidelines 1-10.
2. Attin T (2001). Die Aufhellung verfärbter, avitaler Zähne mit der "Walking-bleach-Technik".
3. Attin T (1998). Sicherheit und Anwendung von carbamidperoxidhaltigen Gelen bei
Bleichtherapien. DZZ 53:1;11-16.
4. Attin T, Burgmaier GM et al (2001). Neues zur Zahnaufhellung mit carbamidperoxidhaltigen
Gelen. ZM 91:5;32-36.
5. Attin T, Hickel R et al (2001). Bleichen von verfärbten Zähnen. Gemeinsame
Stellungnahme der DGZMK und der DGZ. DZZ 56:2;72-73.
6. Bardwell D, Papathanasiou A (2000). A Clinical Study Evaluating the Efficacy of a New
Chairside and Take-Home Bleaching System – Final internal report to DENTSPLY.
January 27, 2000.
7. Barnes DM, Kihn PW, Romberg E, George D, DePaola L, Medina E (1998). Clinical
evaluation of a new 10% carbamide peroxide tooth-whitening agent. Compend Contin Educ
Dent 19:10;968-972,977-978.
8. Christensen GJ (2001). At home tooth bleaching, state-of-art 2001. CRA, 9:4.
9. Clinical Research Associates (1997). Bleichen von Zähnen, State-of-the-Art '97. CRA
10. Clinical Research Associates (2000). Bleichung vitaler Zähne in der zahnärztlichen Praxis.
CRA News (D) 8:6;1-3.
11. Clinical Research Associates (1997). Tooth bleaching, state-of-art '97. CRA News 21:4;1-
12. Ernst CP, Willershausen B, Köttgen C (2000). Bleaching - the state of the art. Submitted
for publication.
13. Floyd RA (1997). The effect of peroxides and free radicals on body tissues. JADA
14. Friedman S (1997). Internal bleaching: long-term outcomes and complications. JADA
15. Glockner K, Ebeleseder K, Städtler P (1997). Das Bleichen von verfärbten Frontzähnen.
Schweiz Monatsschr Zahnmed 107:5;413-420.
16. Goldstein RE, Garber DA (1995). Complete dental bleaching. Quintessence Publishing Co,
17. Gropper G (2000). Ein neues Material für das Home-Bleaching. DS 20:6;28-30.
18. Haywood VB (2000). Current status of nightguard vital bleaching. Compend Contin Educ
Dent 21:Suppl 28;S10-S17.
19. Haywood VB (1997). Nightguard vital bleaching: current concepts and research. JADA
20. Heymann HO, Goldstein RE, Haywood VB, Freedman G (1997). Bleaching of vital teeth.
Quintessence Int 28:6;420-427.
21. Kihn P W, Barnes D M, Adachi E (2000). Clinical Evaluation of a 15% In-Office Hydrogen
Peroxide Tooth Whitening Touch Up Agent - Updated Internal Report to DENTSPLY. April
22. Kihn PW, Barnes DM, Romberg E, Peterson K (2000). A clinical evaluation of 10 percent
vs. 15 percent carbamide peroxide tooth-whitening agents. JADA 131:10;1478-1484.
23. Leonard Jr RH (2000). Nightguard vital bleaching: Dark stains and long-term results.
Compend Contin Educ Dent 21:Suppl 28;S18-S27.
24. Li Y (1997). Toxicological considerations of tooth bleaching using peroxide-containing
agents. JADA 128:4;31S-36S.
25. Li Y (2000). Peroxide-containing tooth whiteners: An update on safety. Compend Contin
Educ Dent 21:Suppl 28;S4-S9.
26. Matis BA, Cochran MA, Eckert G, Carlson TJ (1998). Wirksamkeit und Sicherheit eines
Gels zum Bleichen vitaler Zähne. Quintessenz 49:10;979-987.
27. Matis BA, Cochran MA, Eckert G, Carlson TJ (1998). The efficacy and safety of a 10%
carbamide peroxide bleaching gel. Quintessence Int 29:9;555-563.
28. Müller P (1901). Frühkindliche Zahnverfärbungen aus pädiatrischer Sicht. ZM 91:11;32-
29. Nathoo SA (1997). The chemistry and mechanisms of extrinsic and intrinsic discoloration.
JADA 128:6S-10S.
30. Price RBT, Sedarous M, Hiltz GS (2000). The pH of tooth-whitening products. J Can Dent
Assoc 66:8;421-462.
31. Sherman J (2000). In-House Whitening Case Study FP 084 – Internal Report. February 4,
32. Smidt A, Weller D, Roman I, Gedalia I (1998). Effect of bleaching agents on
microhardness and surface morphology of tooth enamel. Am J Dent 11:2;83-85.
33. Spouge JD (1973). Oral Pathology. The C. V. Mosby Company.
34. Swift Jr EJ, May Jr KN, Wilder Jr AD, Heymann HO, Wilder RS, Bayne SC (1997). Six-
month clinical evaluation of a tooth whitening system using an innovative experimental
design. J Esth Dent 9:5;265-274.
35. Viscio D, Gaffar A, Fakhry-Smith S, Xu T (2000). Present and future technologies of tooth
whitening. Compendium 21:Suppl.28;S36-S43.
Source: http://www.dentsply.at/bausteine.net/f/6454/TM_Illumine.pdf?fd=2
Annotation and Extraction of Relations from Italian Medical Records Giuseppe Attardi, Vittoria Cozza, Daniele Sartiano Dipartimento di Informatica Università di Pisa Largo B. Pontecorvo, 3 I-56127 Pisa, Italy Abstract. We address the problem of extracting knowledge from large scale clinical records written in Italian by physicians. We perform recognition of rel-evant entities such as symptoms, diseases, treatments, measurements, drugs and so forth, and then we determine their semantic relations. We developed suitable training corpora in order to apply machine learning techniques to this task. We report on experiments performed on medical data provided in the context of a regional research project on technologies for health care.
In dieser Ausgabe Aus dem Gemeinderat Ergebnis Nationalratswahl Zugestellt durch Post.at 36. Jahrgang, Nummer 210, 30. Oktober 2013 An einen Haushalt der Gemeinde Assling - Amtliche Mitteilung Umfahrung Mittewald offiziell eröffnet 2,4 km langes Strassenstück in Rekordbauzeit von nur 15 Monaten errichtet Mit einem Festakt an der neuen Kri-












