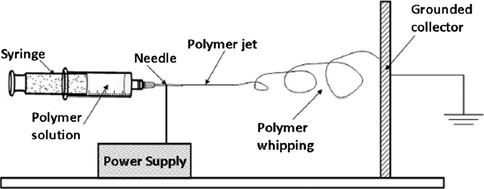
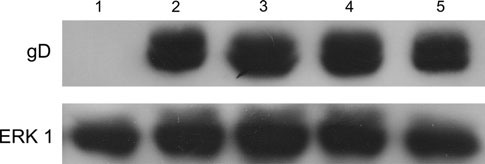
Importancia del agua
Elementalwatson "la" revista ………………. Revista cuatrimestral de divulgación "En el conocimiento y la cultura no Año 4, número 11 sólo hay esfuerzo sino también placer. Llega un punto donde estudiar, o investigar, o Universidad de Buenos Aires Ciclo Básico Común (CBC) aprender, ya no es un esfuerzo y es puro