
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Microsoft word - ijabbr-2014-1017





Available online at h
International journal of Advanced Biological and Biomedical Research
Volume 2, Issue 1, 2014: 110-116
Investigation on the using of linco-spectin solution for in ovo administration in
chicken embryo
Hadi Tavakkoli1*, Amin Derakhshanfar2, Sajedeh Salandari3
1*Department of Avian Medicine, School of Veterinary Medicine, Shahid Bahonar University of Kerman, Kerman, Iran 2 Department of Pathobiology, School of Veterinary Medicine, Shahid Bahonar University of Kerman, Kerman, Iran 3 School of Veterinary Medicine, Shahid Bahonar University of Kerman, Kerman, Iran
Abstract
Nowadays antibiotics are used on a large scale in veterinary and human medicine to cure or prevent
diseases. Some antibiotics injected into hatching eggs to eliminate pathogens and prevention of egg
transmission of disease. Adverse effects of drugs have always been a major concern. There is scantly
information available about the safety and pathological alterations of lincosamide-aminocyclitol
compounds in embryo. The objective of this study was to investigate using of various dosages of Linco-
spectin solution for in ovo administration in chicken embryo. Fertile chicken eggs were divided into six
equal treatment groups as follows: group 1: uninjected group. Group 2: needle-injected group; the needle
was inserted into the yolk sac without any injection. Group 3: phosphate buffered saline-injected group;
whose individuals were injected with phosphate buffered saline. Groups 4, 5 and 6 whose individuals
were injected with linco-spectin solution at a dosage of 10 mg lincomycin and 20 mg spectinomycin or 15
mg lincomycin and 30 mg spectinomycin or 20 mg lincomycin and 40 mg spectinomycin per Kg egg-
weight, respectively. Embryos were re-incubated post-treatment and allowed to develop until day 18 after
which; they were examined for macroscopic and microscopic lesions. Results showed that embryos were
normal in all treatment groups. Microscopically, no lesions were also diagnosed in tissues. Based on
macroscopic and microscopic findings, it is concluded that linco-spectin at above-mentioned
concentration is not toxic for the chicken embryo. So, linco-spectin egg-injection can be used to eliminate
pathogens and prevention of egg transmission of the disease without any adverse effect.
Key words: Chicken, Egg, Embryo, Linco-spectin, Pathology
Introduction
Antibiotics have been used across the globe for many years. Today, they are used on a large scale and are
applied for different purposes (Gharaibeh and Al-Rashdan 2011; Dubb 2012; Garner et al. 2013). In
veterinary medicine, they are used to prevent disease, cure animals and birds, or as a feed additive to
promote growth. Lincosamides are broad spectrum antibiotic and is indicated in treating serious
infections due to susceptible strains of streptococci, pneumococci, and staphylococci. They often dispense
as an aid in preventing chronic respiratory disease associated with Mycoplasma and coliform infections in
chickens (Swayne et al. 2013). Lincosamides bind to the 50S subunit of the bacterial ribosome, and
inhibit the early stages of protein synthesis. The action of lincosamides is mainly bacteriostatic(Sweetman
Corresponding Author E-mail: [email protected] 110 Page
Tavakkoli et al. Int J Adv Biol Biom Res. 2014; 2(1):110-116
et al. 2009; Ahrens and Martin 2013). Linco-spectin is a combination of lincomycin and spectinomycin. Prophylactic application of linco-spectin during the first 3-5 days after hatching decrease the mortality rate in growing chicken. Birds treated with this product show better body weight gains and better feed utilization than non-treated ones (Ahrens and Martin 2013; Swayne et al. 2013). Its use should also be reserved for penicillin-allergic patients or other patients for whom a penicillin product is inappropriate (Ahrens and Martin 2013). In hatcheries, the hygienic process in association with injecting antibiotics into the egg, result in eliminating infection and preventing egg transmission of pathogens. Alternatively, eggs may be dipped in the antibiotic solution for controlling the disease transmission before hatching (Swayne et al. 2013). Adverse effects of drugs have always been a major concern. There is little research in the literature describing the effect of antibiotics on the developing bird embryos, and further studies still need to be undertaken to determine the safety, toxicity and teratogenic potential of antibiotics. Therefore, the aim of this study was to investigate the safety and pathological alterations following the use of various dosages of linco-spectin solution in chicken embryo. We believe that results in this study will contribute to our better understanding of safety and toxicopathological effects of lincosamides and aminoglycosides on the bird embryos.
Materials and Methods
Hatching eggs
Fertile chicken eggs (Ross 308) with the average egg-weight of 50 ± 0.4g and with the same age were
purchased from a local breeder farm. In this farm, birds were kept and grown up under the standard
condition of breeding.
Drugs
Linco-spectin injectable solution was obtained from Razak pharmaceutical company, Iran. Each milliliter
of drug contains 50 mg lincomycin hydrochloride and 100 mg spectinomycin sulfate. It was diluted in
phosphate buffered saline solution. A volume of 0.3 mL of phosphate buffered saline solution with 10 mg
lincomycin and 20 mg spectinomycin or 15 mg lincomycin and 30 mg spectinomycin or 20 mg
lincomycin and 40 mg spectinomycin was inoculated per Kg egg-weight.
Experimental protocol
Eggs were incubated at 37.7ºC and 60% relative humidity. The eggs were randomly assigned to six equal
treatment groups, 10 eggs each, as follows: group 1: uninjected group; embryonated eggs do not receive
any treatment at all. Group 2: needle-injected group, the needle (22-gauge) was inserted into the yolk sac
without any injection. Group 3: phosphate buffered saline injected group, embryonated eggs were injected
with sterile phosphate buffered saline of 0.3 ml/egg into the yolk sac. On day 4 of incubation, the eggs of
groups 4, 5 and 6 were treated with linco-spectin injectable solution at a dosage of 10 mg lincomycin and
20 mg spectinomycin or 15 mg lincomycin and 30 mg spectinomycin or 20 mg lincomycin and 40 mg
spectinomycin per Kg egg-weight, respectively. Embryos received treatment by direct injection into the
yolk sac according to the standard techniques (Hamburger 1942). Embryos were re-incubated post-
treatment and allowed to develop. The viability of the embryos was checked throughout the incubation
period by candling. All embryos were necropsied on day 18 of incubation and examined for macroscopic
and microscopic lesions. The experiment was conducted at the Shahid Bahonar University of Kerman,
Iran. The Latitude and longitude of Kerman are 30°15'N, 56°58'E. The treatments protocols and
procedures in this study were conducted according to local ethical guidelines, and were approved by the
Animal Ethics Committee of the Research Council of Shahid Bahonar University, Iran.
Tavakkoli et al. Int J Adv Biol Biom Res. 2014; 2(1):110-116
Pathological examination
At the end of experiment, on day 18, embryos were humanely killed by placing on ice and then eggs were
opened at the wider end (Jacobsen et al. 2012). After washing in normal saline solution, embryos were
observed under stereomicroscope to study any gross abnormalities on the external body surface. The
membranes and yolk sac were also inspected. Then, the tissues of embryos were dissected out and fixed
in 10% neutral buffered formalin. Following routine preparation of tissues, serial sections of paraffin
embedded tissues of 5 µm thicknesses were cut using a microtome (Slee-Germany) and stained with
hemotoxylin and eosin and studied under light microscope.
Statistical analysis
Statistical analysis was performed using SPSS version 20. The Chi-Squar test was used to determine the
significant differences in lesion occurrence between experimental groups. A P-value of <0.05 was
considered as statistically significant.
Results
Macroscopic results
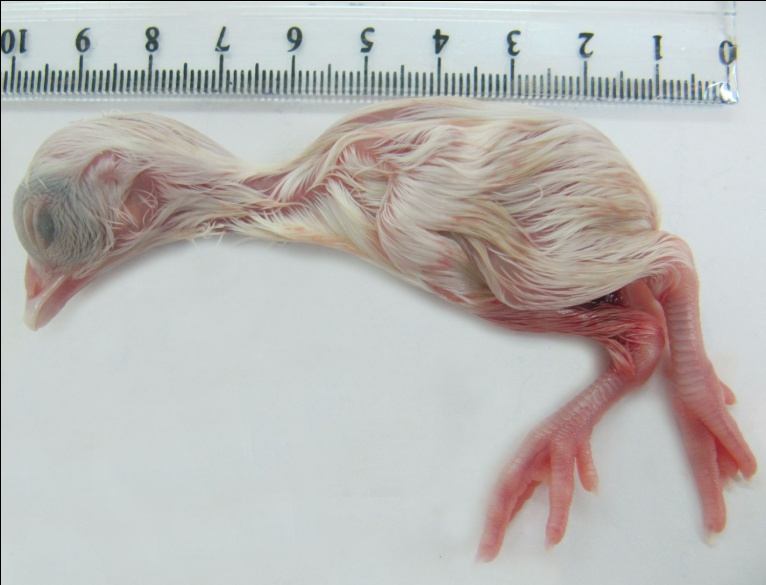
The tissues of the embryos were normal in groups 1, 2 and 3. In linco-spectin-injected groups, groups 4, 5
and 6, there was not any gross abnormality in the tissues and external body surfaces (figure 1). The
obtained tissue samples of these embryos were sent to the pathology laboratory.
Fig. 1. The chicken embryo treated with linco-spectin solution into the yolk sac. The embryo is normal
with no gross lesions.
Microscopic findings
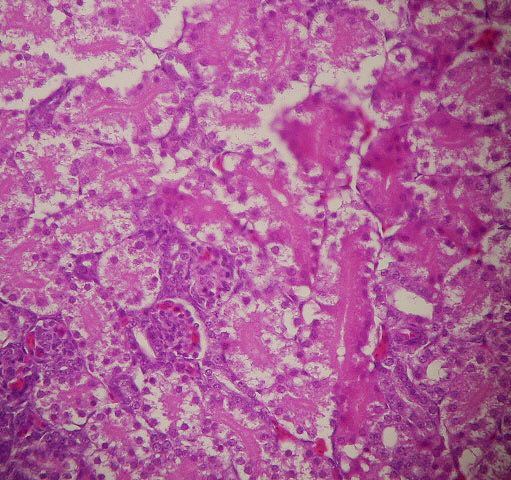
Histopathological evaluation has been revealed that all organs were normal in groups 1, 2 and 3. In
embryos of groups 4, 5 and 6, which received linco-spectin injectable solution, all microscopic structures
were also normal (figures 2-5).

Tavakkoli et al. Int J Adv Biol Biom Res. 2014; 2(1):110-116
Fig. 2. Photomicrograph of the chicken embryo treated with linco-spectin solution into the
yolk sac. A normal kidney tissue is seen. ×100 H&E
Fig. 3. Photomicrograph of the chicken embryo treated with linco-spectin solution into the
yolk sac. The normal structure of the liver with one of the portal areas is seen (arrow). ×100
H&E
Tavakkoli et al. Int J Adv Biol Biom Res. 2014; 2(1):110-116
Fig. 4. Photomicrograph of the chicken embryo treated with linco-spectin solution into the
yolk sac. The normal structure of the heart is seen (arrow). ×100 H&E
Fig. 5. Photomicrograph of the chicken embryo treated with linco-spectin solution into the
yolk sac. A normal lung tissue is seen. ×100 H&E
Discussion
Lincosamide-aminocyclitol compounds have an increased role as therapeutic agents against multidrug-
resistant pathogens. They have a rapid bactericidal effect and a wide antibacterial spectrum. Most gram-
positive and gram-negative organisms are susceptible (Sweetman et al. 2009; Ahrens and Martin 2013).
In many countries such as, United States, Austria, Polish, Canada, Denmark, Germany, Spain, France,
Turkey, Africa and China lincosamide-aminocyclitol compounds have been used successfully for several
decades (Agunos et al. 2012; Persoons et al. 2012; Rasschaert et al. 2012; Garner et al. 2013). In some
countries, this drug may only be approved for veterinary use (Ahrens and Martin 2013). There is little
information available about the effects of injecting linco-spectin solution into the chicken eggs. Besides,
determining the side effects of drugs on the development of chicken embryo is a useful method for
studying the biological properties of drugs. Thus, in this study we investigated the using and safety of
various dosages of linco-spectin solution for in ovo administration in chicken egg. Lesions and organ
injuries following administration were also inspected. On the other hands, this research can further help
us to investigate the toxic potential of lincosamide-aminocyclitol drugs in the human fetus, since the
embryogenesis in chick is similar to human beings (Singroha et al. 2012). Up to now, antibiotic-egg-
treatment has been examined and described in different situations (Ghazikhanian et al. 1980; Sheeks and
Sheeks 1992; Kleven 2008; Singroha et al. 2012; Singroha et al. 2013). The results of these studies show
that injecting antibiotics into hatching eggs can eliminate pathogens and prevent vertical transmission of
disease. Some antibiotics such as tylosin and gentamicin were effective in reducing egg-transmission of
infection (Nascimento et al. 2005). Tylosin was used because of its efficiency against mycoplasmas and
gentamicin was used because of its broad-spectrum activity against bacteria and its low toxicity to host
cells. Dosage and the rout of injection can have an influence on the outcome. For example tylosin can be
toxic for eggs when used in high doses (Nascimento et al. 2005). On the other hand, some injection sites
that are present in fertile eggs at day 4 of incubation are the air cell and yolk sac. Injection antibiotics into
the air cell of the egg is discontinued and is not suitable for breeding purposes because drastic mortality
of embryos occur when eggs treat by this procedure (McCapes et al. 1977; Nascimento et al. 2005). Our
results obviously showed no gross abnormality in the tissues and external body surfaces of embryos
exposed to various dosages of the linco-spectin solution by yolk sac rout. Histopathological examination
has also been revealed that all organs were normal in embryos. Therefore, these results suggest that the
best linco-spectin injection sites in ovo may be the yolk sac.
Tavakkoli et al. Int J Adv Biol Biom Res. 2014; 2(1):110-116
Conclusion
Based on macroscopic and microscopic findings, it is concluded that linco-spectin solution can be used
for the success of the eradication scheme with low toxicity to chicken embryo. In addition, the yolk sac is
an appropriate site for injecting antibiotic drugs.
Acknowledgment
The authors wish to thank Mr. S. Hasanzadeh for his kind cooperation in slide preparation.
References
Agunos A, Léger D, Carson C (2012). Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. The Canadian Veterinary Journal 53(12): 1289.
Ahrens FA, Martin RJ (2013). Antimicrobial drugs. Handbook of Veterinary Pharmacology 347.
Dubb A (2012). Antibiotic Update. South African Family Practice 4(5).
Garner DL, Evans KM, Seidel GE (2013). Sex-Sorting Sperm Using Flow Cytometry/Cell Sorting.
Spermatogenesis, Springer: 279-295.
Gharaibeh S, Al-Rashdan M (2011). Change in antimicrobial susceptibility ofMycoplasma gallisepticumfield isolates. Veterinary microbiology 150(3): 379-383.
Ghazikhanian GY, Yamamoto R, McCapes R, Dungan WM, Larsen C, Ortmayer H (1980). Antibiotic Egg Injection to Eliminate Disease II. Elimination of Mycoplasma meleagridis from a Strain of Turkeys. Avian Diseases 48-56.
Hamburger V (1942). A manual of experimental embryology, University of Chicago Press Chicago.
Jacobsen ID, Große K, Hube B (2012). Embryonated Chicken Eggs as Alternative Infection Model for
Pathogenic Fungi. Host-Fungus Interactions, Springer: 487-496.
Kleven S (2008). Control of avian mycoplasma infections in commercial poultry. Avian Diseases 52(3): 367-374.
McCapes R, Yamamoto R, Ghazikhanian G, Dungan W, Ortmayer H (1977). Antibiotic Egg Injection to Eliminate Disease I. Effect of Injection Methods on Turkey Hatchability and Mycoplasma meleagridis Infection. Avian Diseases 57-68.
Nascimento ER, Pereira V, Nascimento M, Barreto M (2005). Avian mycoplasmosis update. Revista Brasileira de Ciência Avícola 7(1): 1-9.
Persoons D, Dewulf J, Smet A, Herman L, Heyndrickx M, Martel A, Catry B, Butaye P, Haesebrouck F (2012). Antimicrobial use in Belgian broiler production. Preventive Veterinary Medicine 105(4): 320-325.
Rasschaert G, Michiels J, Arijs D, Wildemauwe C, De Smet S, Heyndrickx M (2012). Effect of farm type on within-herd Salmonella prevalence, serovar distribution, and antimicrobial resistance. Journal of Food Protection 75(5): 859-866.
Tavakkoli et al. Int J Adv Biol Biom Res. 2014; 2(1):110-116
Sheeks OB, Sheeks RL (1992). Egg injection method, apparatus and carrier solution for improving hatchability and disease control, Google Patents.
Singroha R, Srivastava S, Chhikara P (2012). Effect of Gentamicin on kidney in developing chicks. Eur J Anat 16(2): 119-126.
Singroha R, Srivastava S, Chhikara P (2013). Effect of gentamicin on proximal convoluted tubules of kidney in developing chicks. Journal of the Anatomical Society of India 62(1): 17-22.
Swayne DE, Glison LR, McDonald LR, Nolan LK, Suarez DL, Nair V (2013). Disease of poultry, A John Wiley and Sons publication.
Sweetman SC, Pharm B, PharmS F, Eds. (2009). Martindale: The Complete Drug Reference. London, Pharmaceutical Press.
Source: http://www.ijabbr.com/pdf_7040_0f4a298dff69ba2a74b9ee6975bb00dd.html
Top 10 Global Consumer Trends for 2016 Not to be distributed without permission. TOP 10 GLOBAL CONSUMER TRENDS Daphne Kasriel-Alexander Consumer Trends Consultant © EUROMONITOR INTERNATIONAL 2016 © EUROMONITOR INTERNATIONAL © EUROMONITOR INTERNATIONAL © EUROMONITOR INTERNATIONAL Consumption in 2016 is an interesting blend of established and new trends with
In dieser Ausgabe Aus dem Gemeinderat Ergebnis Nationalratswahl Zugestellt durch Post.at 36. Jahrgang, Nummer 210, 30. Oktober 2013 An einen Haushalt der Gemeinde Assling - Amtliche Mitteilung Umfahrung Mittewald offiziell eröffnet 2,4 km langes Strassenstück in Rekordbauzeit von nur 15 Monaten errichtet Mit einem Festakt an der neuen Kri-












