
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Ijrpc.com
IJRPC 2014, 4(4), 972-976 M Phatak et al. ISSN: 2231
2781
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY
Available online at
Research Article
DEVELOPMENT AND VALIDATION OF A HIGH PERFORMANCE
LIQUID CHROMATOGRAPHY METHOD FOR THE SIMULTANEOUS
QUANTIFICATION OF ALBENDAZOLE AND CLOSANTEL FROM
VETERINARY FORMULATION
M. SPhatak*, VV. Vaidya and H. MPhatak
Department of Chemistry, Ramnarain Ruia College, Matunga,
Mumbai-400019, India.
ABSTRACT
A simple and rapid Reverse Phase HPLC method has been developed for the simultaneous
quantification of Closantel and Albendazole from veterinary anthelmintic formulation. HPLC analysis
was performed on C18 column maintained at 30ºC using a simple mixture of acetonitrile, distilled
water and methanol as isocratic mobile phase at a flow rate of 1.8ml per minute at detection
wavelength of 254nm. The method was validated for accuracy, precision, linearity, specificity and
sensitivity in accordance with International Conference on Harmonization guidelines. Good linear correlation coefficients (r2>0.999) were obtained for calibration plots in the range of 50–150μg/ml
for Closantel and 25 – 75 μg/ml Albendazole. Intraday and Interdayprecision of retention times and
peak areas were less than 2.0%. Accuracy of the method was between 99.35% and 99.86% for
Closantel and 99.58% and 100.16% for Albendazole. Validation revealed the method to be specific,
accurate, precise, reliable and reproducible. The method was successfully used for quantitative
analysis of these analytes from marketedVeterinary anthelmintic formulation.
Keywords: Simultaneous determination; RP-HPLC; Albendazole; Closantel; veterinary formulation.
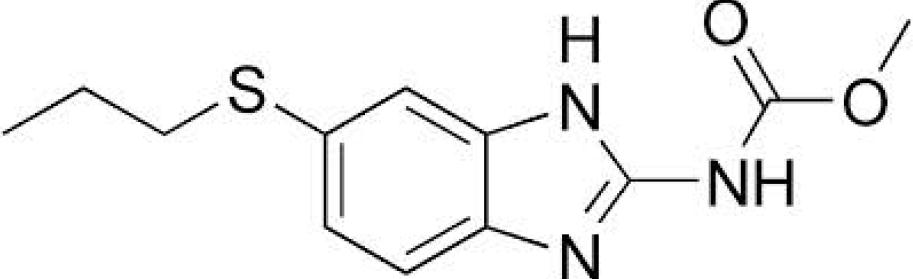
analytical methods used were LC MS/MS and
anthelmintic agent (Figure 1). It is a broad-
Albendazole is a benzimidazole class of drug
spectrum antiparasitic agent used against
being used in the treatment for parasitic
several species and developmental stages of
infections (Figure 2). It is a broad spectrum
trematodes, nematodes and arthropods. The
anthelmintic effective against roundworms,
anti-trematode activity of closantel is mainly
tapeworms, and flukes of domestic animals
used against liver fluke. The anti-nematode
and humans4. Albendazole is available
and anti-arthropod activity is especially used
commercially in the form of tablets for human
against those species which feed on blood or
use and tablets and oral drench for veterinary
plasma. Closantel has shown beneficial effect
use. Albendazole is used alone or in
when used in humans for the treatment of
combination with other anthelmintic drugs for
better efficacy. Various analytical method have
Closantel is available commercially as oral
treatments in the form of tablets and drench
Albendazole5,6,7.
formulation alone or in combination with other
There is no method available currently for the
anthelmintic agents for veterinary use. There
determination of Closantel and Albendazole
are a few methods available for the
from a veterinary formulation. In the present
quantification of Closantel from biological
work the development and validation of a
matrix2 or pharmaceutical presentation3. The
simple and rapid method for the simultaneous
IJRPC 2014, 4(4), 972-976 M Phatak et al. ISSN: 2231
2781
quantification the two drugs from an oral
Calibration Curve
drench formulation.
Standard solutions of 50 - 150 μg/mL of
Closantel and 25-75 μg/mL of Albendazole
MATERIALS AND METHODS
were analyzed to check the linearity range.
Instruments
Specificity
Technologies 1200 HPLC model with photo
The specificity of the method was ascertained
diode array detector, Rheodyne injector having
by analysing the standards and the samples.
20μL loop volume. Separation was carried out
The peaks of Closantel and Albendazole in
using Thermo Electron Corporation, Hypersil
sample were confirmed by comparing the
BDS C18 (150 x 4.6 mm, 5μ) column.
retention time and spectra of the standards8.
Detection was carried out using UV-visible
detector. The flow rate was kept at 1.8 ml/ min
Precision
and the column oven was maintained at 30 ºC.
Precision was evaluated by injecting six
The total chromatography run time was 4
injections at the working level concentrations
of Closantel (100μg/mL) and Albendazole
(50μg/mL) were analysed to examine the
Reagents
precision of the method. Intraday precision
All chemicals and solvents used were of HPLC
and interday precision for the developed
grade. Distilled water generated from TQA
methods were measured in terms of %RSD.
The % RSD of the six replicates was
Closanteland Albendazole were provided as
considered for intraday precision and % RSD
gift sample by Cipla Ltd., India. Veterinary
of six replicate injections, injected on two
anthelmintic formulation Closal drenchwas
different days were considered for inter day
purchased from local market.
precision. The concentration values for both
intraday precision and interday precision were
Standard Solutions
calculated and percent relative standard
Stock solutions, of 1 mg/ml of Closantel and
deviation were calculated using following
0.5 mg/ml of Albendazole were freshly
prepared individually in methanol. Aliquots of
Closantel (50-150μg/mL) and Albendazole
% RSD = [S/X] 100,
(25-75 μg/mL) were prepared by subsequent
dilutions of the stock solutions in the mobile
S is standard deviation and
X is mean of the sample analyzed.
Sample Preparation
Accuracy
The Closal drench is available as a formulation
Accuracy of the method was determined by
containing 19 g/L of Albendazole and 37.5 g/L
recovery experiments. Recovery experiments
of Closantel. The sample for analysis was
were carried out by the standard addition
prepared bydiluting 1.1 ml of the formulation to
method. This study was performed by diluting
100 ml with mobile phase. Further, 5 ml of this
adding 10 ml, 10 ml and 20 ml each of the of
solution is diluted to 20 ml to give the sample
standard solution of Closantel (1000 μg/ml)
and Albendazole (500 μg/ml) to three different
volumetric flasks containing the 1.1 ml of
sample solution has a concentration of 103.1
sample. The resultant sample solutions were
correspondingly diluted to provide 50%, 100%
and 150% of Closantel and Albendazole
concentration compared to the standard
Preparation of Mobile Phase
solution. The amounts of standard recovered
The mobile phase is prepared by mixing
were calculated in terms of mean recovery
acetonitrile, distilled water and methanol. In a
with the upper and lower limits of percentage
volumetric cylinder 600 ml of acetonitrile is
relative standard deviation8.
taken to which 300 ml of distilled water and
100 ml of methanol is added. The mobile
RESULTS AND DISCUSSION
phase is sonicated for 2 minutes and filtered
Optimization of the method was carried out
through 0.45 μm Teflon filter before using in
the HPLC system.
acetonitrile,distilled water and methanol. A
solvent combination of acetonitrile: D.W.:
methanol (60:30:10) gave a satisfactory
separation of the Closantel and Albendazole.
This optimized mobile phase separated
IJRPC 2014, 4(4), 972-976 M Phatak et al. ISSN: 22312781
Albendazole at 1.47 min and Closantel at 2.21
solution is shown in figure 5. Even though
min respectively. The column efficiency,
Closantel and Albendazole show common
resolution and peak asymmetry and resolution
therapeutic activities, they are entirely different
were calculated for the standard solutions and
in their chemical nature. Good resolution of
are presented in Table 1.Increasing the
Closantel and Albendazole in a simple
concentration of distilled water in the solvent
isocratic mobile phase were achieved. The
system resulted in deterioration of the peak
shape and retention of peaks on the column,
sample purchased were done and the results
resulting is longer analysis time. On the other
demonstrated consistency between different
hand increasing the organic phase caused the
samples and analysis conducted on different
Closantel peak to elute very close to the
placebo peaks. The calibration curves of
Closantel and Albendazole were linear in the
CONCLUSION
range of 50- 150μg/mL for Closantel and 25-
Based on the above results it can be
75 μg/mL for Albendazole. The linearity
concluded that the developed method can be
experiment observations and the slope, y-
used for the simultaneous quantification of
intercept and regression coefficient (r2) are
Albendazole and Closantel for routine quality
shown in Table 2.
control analysis.
Accuracy rom sample matrices was between
99.52% &100.10% and 99.67% and 99.98%
respectively for Closantel and Albendazole.
We would like to thank Cipla Ltd., India for
The individual standard chromatogram for
providing the analytical standards for the
Closantel and Albendazoleare shown in figure
3 and 4, while the chromatogram of sample
Fig. 1: Structure of Closantel
Fig. 2: Structure of Albendazole
IJRPC 2014, 4(4), 972-976 M Phatak et al. ISSN: 22312781
Fig. 3: Typical chromatogram for Closantel
Fig. 4: Typical chromatogram for Albendazole
Fig. 5: Typical chromatogram for sample solution
IJRPC 2014, 4(4), 972-976 M Phatak et al. ISSN: 22312781
Table 1: Chromatographic peak characteristics
Parameter
Albendazole
Closantel
Theoretical plates
Table 2: Linearity experiment observations
Albendazole
Closantel
Linearity level
Peak area
Peak area
REFERENCES
albendazole and metabolites in human
1. Christian Gloeckner. Repositioning of
plasma for clinical pharmacokinetic
an existing drug for the neglected
studies. J Pharm Biomed Anal.
2002;15;30(3):801-13.
Proceedings of National Academy of
Science of the United States of
America. 107(8):3424–3429.
2. Hanwen Sun. Validated method for
method for simultaneous estimation of
determination of ultra-trace Closantel
albendazole and ivermectin in tablet
residues in bovine tissues and milk by
dosage form, Indian J ChemTechnol.
2008;15:617-620.
7. Shreya Shah, Dey S, Prasanna
ionization-tandem mass spectrometry.
2007;1175:227–233.
3. Gayatri S. Method development and
validation for estimation of Closantel in
albendazole and praziquantel in bulk
tablet dosage form by RP-HPLC
and in a synthetic mixture, Journal of
2011;4:246‐251.
8. International
4. Theodorides VJ, Gyurik RJ, Kingsbury
WD and Parish RC. Antihelmintic
Activity of AlbendazoleAgainst Liver
Pharmaceuticals for Human Use, ICH
Gastrointestinal
Guideline‐Validation
Experientia. 1976;32(8):702–703.
Procedures: Text and Methodology
Q2 (R1), Current Step 4 version,
Fleckenstein L. HPLC assay for
Source: http://www.ijrpc.com/files/29-4206.pdf
Allegato a D.G.R. n. 2-5947 del 28.05.2007 – aggiornato con D.D. 433 del 05.07.2010 Le misure preventive in caso di temperature Protocollo operativo Raccomandazioni per il personale sanitario (Le sezioni riferite alle raccomandazioni sono tratte dall'opuscolo "Le misure preventive in caso di temperature elevate – Raccomandazioni per il personale sanitario", Torino 2004, a cura di: Cristiana Ivaldi, Ennio Cadum, Elena Coffano, Moreno Demaria, Lidia Fubini,
Guidelines for the Use of Mifepristone for Medical Abortion in New Zealand Abbreviations used in this document.61. Introduction.72. Background.7 2.1 Approval of mifepristone (Mifegyne) in New Zealand.72.2 Legal requirements.7 3. Second Trimester Abortion.8 3.1 Background.83.2 Mifepristone dosage.83.3 The prostaglandin.83.4Interval between mifepristone and the prostaglandin.93.5 Second trimester protocols.9





