
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Microsoft word - 1960 order
BEFORE CONTROLLER OF PATENTS
THE PATENT OFFICE, DELHI
In the matter of pre-grant opposition by
way of representation under section 25(
1) of The Patents Act, 1970 as amended
by The Patents (Amendment) Act, 2005
In the matter of rule 55 of The Patents
Rules, 2003 as amended by The Patents
(Amendment) Rules, 2006
In the matter of Application No:
APPLICANT: BAYER HEALTHCARE AG, GERMANY.
Present in hearing:
Dr. Sanjay Kumar (Agent representing the Applicant)
OPPONENT I: NATCO PHARMA LIMITED, HYDERABAD.
Hearing held on 24th December, 2014
Present in hearing:
Dr.Chitra Anand (Agent representing the Opponent)
OPPONENT II: FRESENIUS KABI ONCOLOGY LIMITED,
GURGAON,
Hearing held on 05th February, 2015.
Present in hearing:
Mr. Vishal Sudan (
Agent representing the Opponent).
Dr.A.P.Singh (Examiner of Patents & Designs)
An application for a patent bearing number
1960/DELNP/2007 was filed in
Patent Office, Delhi on 14th
March, 2007 entitled
"THERMODYNAMICALLY STABLE FORM OF A TOSYLATE SALT".
A request for examination under Section 11-B was filed on 22nd September,
2008 and was assigned a Request No. 9316/RQ-DEL/2008. As per the
provision under Section 11-A of Patents Act, the said application was
published on 17th August, 2008.
The said application was examined according to the provisions in force of
the Patents Act, 1970 (as amended) and First Examination Report
(herein after called as FER) was issued to the Applicant's Agent on
1 6 t h September, 2013.The applicant filed reply to FER on 04th
March, 2014 with amended set of claims.
It is to be noted that two pre-grant oppositions under section 25(1)
were filed a g a i n s t this application, first by
1) Natco Pharma Limited, Hyderabad represented by
R a j e s h w a r i & Associates on 14th October, 2011. The said pre-grant
opposition was forwarded to the applicant on 16th September, 2013 & the
applicant filed reply statement & evidence on 16th December, 2013 within
three months from issuing of pre-grant opposition. Hearing in this matter
was held on 24th December, 2014
(Herein after Opponent I) and second
2) Fresenius Kabi Oncology Limited, Gurgaon represented by the
company itself. The said pre-grant opposition was forwarded to the applicant
on 21st October, 2014 & the applicant filed reply statement & evidence
on 19th January, 2015 within three months from issuing of pre-grant
opposition. Hearing in this matter was held on 05th February, 2015.
(Herein
1960/DELNP/2007
after Opponent II)
Prior to hearing on this matter several dates of hearing to dispose
of the said pre-grant oppositions were fixed by this Office as
adjournments were taken by the applicant several times.
Having dealt with the hearings independently I will discuss the contents
independently & finally decide the matter commonly.
The amended claims as filed on 04th March 2014 which are opposed are
as follows
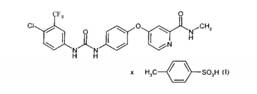
1) A tosylate s a l t of 4-{4-[({[4-chloro-3 (trifluoromethyl) phenyl]
a m i n o } carbonyl) amino] phenoxy}-N-methylpyridine-2- carboxamide
in the polymorph I.
2) A preparation of the compound of the formula (I) in the
polymorph I, which comprises effecting the compound of the
formula (I) in the polymorph II in an inert solvent until
quantitative conversion to the polymorph I.
3) The preparation of the compound of the formula (I) in
the polymorph I as claimed in claim 2, wherein the compound of the
formula (I) in the polymorph II is effected in an inert solvent and
seeded with crystals of the compound of the formula (I) in the
4) The preparation of the compound of the formula (I) in the polymorph
I, wherein the compound of the formula (I) in the polymorph II is heated
to from 195 to 222°C at a heating rate of from 10 to 30°C per minute and
subsequently cooled to from 10 to 30°C at a cooling rate of from 1 to 4° C
5) A pharmaceutical composition comprising the compound of the
formula (I) mainly in the polymorph I and no significant fractions
of another form of the compound of the formula (I).
6) The pharmaceutical composition as claimed in claim 5 containing
more than 90 percent by weight of the compound of the formula
(I) in the polymorph I related to the total amount of the compound of
the formula (I) present in the composition.
7) The pharmaceutical composition as claimed in claim 5 comprising one
or more inert, nontoxic, pharmaceutically suitable excipients.
by dissolving or suspending the compound of the formula (I) in the
polymorph II in an inert solvent and stirring or shaking it until
quantitative conversion to the polymorph I.
9) A compound of the formula (I) as claimed in claim 8 obtainable
by dissolving or suspending the compound of the formula (I) in the
polymorph II in an inert solvent and seeding it with crystals of the
compound of the formula (I) in the polymorph I.
10) A combination comprising the compound o f the formula (I) in the
polymorph I and one or more other pharmaceutical agents.
11) The combination as claimed in claim 10 wherein the one or
more other pharmaceutical agents are cytotoxic agents, signal
transduction inhibitors, anti- cancer agents or antiemetics.
12) The pharmaceutical composition as claimed in one of the claims
5 and 6 comprising one or more other pharmaceutical agents.
wherein t h e one or more other pharmaceutical a g e n t s are
cytotoxic agents, signal transduction inhibitors, anti cancer agent, or
Documents relied by Natco Pharma Limited, Hyderabad (Opponent I)
Before proceeding the opponents has relied on the cited documents which are
1) Representation u/s 25(1) by the Petitioner/Opponent
2) Annexure-A: Specification as mentioned in impugned Indian
N a t i o n a l Phase Application 1960/DELNP/2007.
3) Annexure-B: A printout of WO 00/42012.
4) Annexure-C: A printout of WO 03/068228.
5) Annexure-D: A printout of WO 03/047579.
6) Annexure-E: A printout of article 'Current Pharmaceutical Design
2002. 8, 2255-57' titled 'Bay 43-9006: Preclinical Data' by Scott
7) Annexure-F: A printout of W0/1996/027592.
8) Annexure-G: A printout of W0/1999/00 1444.
I further proceed on the grounds on which the actual opposition is based.
GROUNDS OF OPPOSITION:
lA) Section 3(c): Subject of claims 1-18 of the complete specification is
not patentable under this Act
Claims 1-18 are drawn to polymorphic forms of 4-{4-[({[4-
chloro-3- trifluoromethyl) phenyl] amino} carbony) amino] phenoxy}-N-
methyl pyridine-2-carboxamide, also known as Sorafenib. Sorafenib is a
known substance as admitted by the Applicant in the impugned specification -
the said compound is known from WO 00/42012 (Annexure B), WO
03/068228 (Annexure C) and WO 03/047579 (Annexure D). The Applicant
also admits that a polymorphic form is already prepared in WO 00/42012 and
hence the purported new polymorphic forms of this substance claimed in the
impugned application are at best characterization of certain known properties
of a known substance. Hence claim 1-18, falls squarely within the mischief
and prohibition of Section 3(c) and therefore not patentable at all.
IB) Section 3(d): Subject of claims 1-18 of the complete specification is
not patentable under this Act.
Claims 1-18 are drawn to polymorphic forms of 4-{4-[({[4-
chloro-3- trifluoromethyl) phenyl] amino} carbony) amino] phenoxy}-N-
methyl pyridine-2-carboxamide. The compound a s claimed i n the
impugned a p p l i c a t i o n i s already known as sorafenib and is also
admitted by the Applicant as explained in the foregoing paragraphs.
As per section 3(d) of the Indian Patents Act, 1970, the mere discovery of a
new form of a known substance that does not result in the enhancement of the
known efficacy of the substance is not patentable. The alleged
invention of polymorphic form I, as claimed, is not supported by any
examples or any other data of enhanced efficacy over that of known
substances, including the metastable form.
In the absence of any evidence in the specification and given that the
stable form (form I} is nothing but the stable form of metastable form
disclosed in W000/42012, it must be assumed that the polymorphic form I
as claimed in claim I of the impugned application does not differ
significantly in properties with regard to efficacy as compared to the
polymorphic form II.
Hence the form I as claimed is not patentable under section 3(d) of the
Indian Patent Act of 1970. Moreover the process for the preparation of
sorafenib tosylate polymorph I as claimed in claims 2-4 neither involve any
new reactant nor result in any new product. Thus the claimed invention falls
within the scope and mischief of Section 3 (d)
IC) Section 3(e): Subject of claims 7, 8 and 12 and 15-18 of the complete
specification is not patentable under this Act.
It is submitted that claims 7, 8 and 12 and 15-18 are drawn to a
pharmaceutical compositions, the composition comprises of the polymorphs
of claims 1 along with a pharmaceutically acceptable excipient. However,
there are no examples to indicate that the composition is synergistic. The
specification does not provide any data to substantiate the statement that the
combination as claimed is synergistic. In the absence of such data, the
composition is a mere admixture that results in the aggregation of the
properties of the two known substances. Accordingly, the composition as
claimed is a mere admixture and on this ground alone ought to be
ID) Section 3(i): Subject of claims 5 to 6 and 9 to 11 of the
complete specification is not patentable under this Act.
Claims 5-6 and 9-11 correspond to method of treatment and are not
patentable under sec 3(i). It is submitted that under the act, any method for
treatment of an animal or human cannot be patented. The said claims fall
within the said provision and hence should be rejected.
GROUND II
II) Section 25(1) (b)/(c): Lack of novelty
The invention as claimed in claims 1-18 lacks novelty and are not patentable
under Section 25(1) (b)-(c) of the Patents Act, 1970 (as amended in 2005;
hereinafter referred to as "the Act"). It is submitted that none of the claims
of 1960/DELNP/2007 are novel and they are all liable to be rejected on
this ground alone.
PRIOR USE
It is submitted that sorafenib tosylate is well known and widely used before
the date of the impugned application and hence the polymorph as claimed by
It i s f u r t h e r submitted that sorafenib tosylate (in the form I as claimed
in the impugned application) has been subject of extensive prior use by the
Applicant. Such prior use has been well documented as in Current
Pharmaceutical Design 2002, 8, 2255-57 titled 'Bay 43-9006: Preclinical
Data' by Scott Wilhelm et al.
The said article narrates the use of the compound Bay43-9006 in pre-clinical
settings (Annexure E). The compound would exist and would have been used
naturally in its most stable form i.e. form I. Such documents constitute prior
use of the polymorphic compound claimed. Thus, all claims stand anticipated
by disclosure in prior art, prior claiming and by prior use and
hence this application, ought to be rejected.
GROUND Ill
Ill) Section 25(1) (e): Lack of inventive step
The invention so far as claimed in any claim of the complete specification is
obvious and clearly does not involve any inventive step, having regard to the
matter published as mentioned in clause (b) or having regard to what was
used in India before the priority date of the claim. It is submitted that the
claims of the impugned application are not inventive and obvious.
The impugned application admits at WO 00/42012 that one form of the
polymorph is already disclosed. "The compound of the formula {II} is
prepared in the manner described in WO 00/42012. The compound of the
formula {I} is prepared according to a general standard method for
the preparation of tosylate salts, as described in example I of the
working examples. In this method, the compound of the formula {I) is
obtained in one crystal polymorph which is referred to herein below as
polymorph II. Polymorph I I has a transition point o f 1 9 4 °C a n d a
spectrum, FIR spectrum and NIR spectrum (Tab. 1-6, Fig. 1-6}. It has been
found that polymorph II is metastable." (see para 1 of page 3 of the
impugned specification).
Assuming but not conceding that the impugned application pertains to the
conversion of one polymorphic form to another polymorphic form, it
is submitted that the conversion of one crystalline form to another crystalline
form is known from various prior arts which are in public domain since a
long time and much before the priority date of impugned patent application.
For instance W0/1996/027592(attached herewith as Annexure F) published
on 12 September 1996 teaches process for the preparation of polymorphic B
form of (E)-4-[[3-[2-(4-cyclobutyl-2-thiazolyl) ethenyl] phenyl] amino]-2,
2-diethyl-4-oxobutanoic acid by agitating Polymorphic Form A in a solvent
and adding the seed crystals of Form B.
Also, a disclosure in W0/1999/001444 (attached herewith as Annexure G)
published on 14 January 1999 (Example 23) teaches process for the
preparation of thermodynamically stable polymorphic form I of the
antagonist 2-(r)-(1-(r) -(3,5-bis(trifluoromethyl)
phenyl)ethoxy)-3-(s)-(4-fluoro) phenyl-4-(3-5 (-oxo-1hAh-1,2A,-triazolo)
methylmorpholine by effecting the compound of Form II in methanol and
seeded with crystals of Polymorphic form I.
Thus, the conversion of one polymorphic form of compound to another is
spontaneous and is also known from prior art and is within the routine skills
of a person skilled in the art. Moreover, the use of inert solvent and seeding
technology, which appears to be the crux of claims 2-4 of impugned
application, is also in the public domain.
Claims 2-4 and claims 13-14 of the impugned patent application deal
with process for the preparation of polymorphic form I of sorafenib
13- 14 deal with the process of converting from polymorph II to polymorph I.
"The process involves effecting the polymorphic form II of sorafenib tosylate
in an inert solvent and stirring or shaking until Polymorphic Form I
was obtained".
It is submitted that stirring and shaking are but means to accelerate
the conversion and even without such process, the polymorphic form II is
automatically converted to polymorphic form I. Polymorph-I of
sorafenib tosylate is found to be the most stable crystal form and claimed as
a compound in claim-1 of the impugned application. The pharmaceutical
composition and method of use claims in the said application, all deal with
polymorph-I only as this is 'stable'.
The process and methods used for making polymorphs or converting
a metastable polymorph into a stable polymorph is well documented in
literature. For any a chapter from the book "Advanced Pharmaceutical
Solids" by Jens T. Carstensen, published by Marcel Dekker Inc, 2001 -(see
Annexure H) clearly discloses the various experimentation techniques to
obtain polymorphs and to convert metastable polymorphs into stable
Thus from the above following are established
1. The conversion of one polymorphic form to another polymorphic form
is well known in prior art.
2. The use of the inert solvent and seeding technology for the conversion
one polymorphic form to another polymorphic form is known from the prior
art. Hence, the process of conversion of polymorphs and the resultant
polymorphic form is well known from prior art. On this ground alone all
claims ought to be rejected.
GROUND IV
IV) Section 25(1) (f): Subject of claims 1 to 3 is not an invention within
the meaning of this Act or is not patentable under this Act.
It is submitted that since claims 1-18 are not novel, are not inventive and lack
industrial application, they do not constitute an 'invention' under the Act.
In this regard, the Opponent craves leave to refer and rely on submission
made in Grounds 1-VI above which are not being repeated for the sake of
i) Subject of claims are not patentable under section 2(1) (ja) of this act:
The claimed invention falls under the mischief of Section 2(1)(ja) being
devoid of inventive step as according to definition of inventive step, the
invention should have a technical advancement over the prior art or it
should show economical significance or both and should not be obvious to a
person skilled in the art. The alleged invention as claimed is not a technical
V} Section 25 (l) (g}: The complete specification does not sufficiently
and clearly describe the invention or the method by which it is to be
performed.
It is submitted that the complete specification of 1960/DELNP/2007 does not
describe the invention claimed or the method by which it is be performed.
The particulars thereof are as under:
Claims 1-18 of the application at hand bitterly suffer from lack of adequate
description and are liable to be rejected.
GROUND VI
VII) Section 25(1) (h): The patentee has failed to disclose to the
Controller the information required under Section 8:
It is submitted that the Applicant-Respondent has failed to disclose the
details of corresponding foreign applications filed and on this ground alone
the patent application should be rejected.
The applicant is required to provide all the information regarding
the prosecution of the equivalent applications till the grant of the Indian
application to the Controller in writing from time to time and also within
the prescribed time. The applicant has failed to furnish the details of
National phase applications filed in USA, Europe etc, which are still
under examination and not granted. Therefore the applicant has failed to
comply with the requirements of the section 8 of the act and the opponent
demands rejection on this ground also
Documents relied by Fresenius Kabi Oncology Limited, New-Delhi
(Opponent II)
Documents relied upon
1) Representation u/s 25 (1) by the opponent 1-30.
2) Annexure- I: Claims of 1960/DELNP/2007 31-33.
3) WO 03/068228 (referred to hereinafter as D1) published on August 21,
34-110, 2003; annexed hereto as Exhibit 1.
4) WO 03/050111 (referred to hereinafter as D2) published on Jun 19,
2003; 111-132 annexed hereto as Exhibit 2.
5) A document downloaded from the EMA website (hereinafter referred to
as133-181 D3) which is annexed hereto as Exhibit 3.
6) A document downloaded from the FDA website (hereinafter referred to
as 182-186 D4) which is annexed hereto as Exhibit 4.
7) Chemistry & industry, 1989, pages 527-529 (hereinafter D5) which is
187-189 annexed hereto as Exhibit 5
8) Pharmaceutical Research, vol. 12, No. 7, pages 1995, 945-954
(hereinafter 190-199 D6) which is annexed hereto as Exhibit 6.
9) X-Ray diffraction data of products obtained by repeating (four times) the
200-example 1 of the impugned application are annexed hereto as Exhibit 7
to 203-214 Exhibit 10.
10) X-Ray diffraction data of product obtained by following the method of
D2215-217 is annexed hereto as Exhibit 11.
11) Affidavits of Ms. Sandeep Kaur, who repeated the experiment of example
1, 218-218 of the impugned application is annexed hereto as Exhibit 12
12) Affidavits of Mr.Nikunj Kachhadia, who repeated the expt. of 219- 219
example 1 of the impugned application is annexed hereto as Exhibit 13
13) Affidavit of Mr. Varun Sharma who performed the method as given in
D2 220 is annexed hereto as Exhibit 14
GROUNDS OF OPPOSITION OF OPPONENT II
1. The application is opposed on the following grounds:
a). Section 25(1) (b): Novelty I Anticipation
That the invention so far as claimed in any claim of the complete
specification has been published before the priority date of the claim-
(i) In any specification filed in pursuance of an application for a patent made
in India on or after the 1st day of January, 1912; or
(ii) In India or elsewhere, in any other document: Provided that the
ground specified in sub-clause (ii) shall not be available where such
publication does not constitute an anticipation of the invention by virtue of
sub-section (2) or subsection (3) of section 29;
b). Section 25(1) (d): Prior Knowledge / Prior Use
That the invention so far as claimed in any claim of the complete
specification was publicly known or publicly used in India before the
priority date of that claim.
Explanation.-For the purposes of this clause, an invention relating to a
process for which a patent is claimed shall be deemed to have been publicly
known or publicly used in India before the priority date of the claim if a
product made by that process had already been imported into India before
that date except where such importation has been for the purpose of
reasonable trial or experiment only;
c). Section 25(1) (e): Obviousness I lack of inventive step
That the invention so far as claimed in any claim of the complete specification
is obvious and clearly does not involve any inventive step, having regard to
the matter published as mentioned in clause (b) or having regard to what was
used in India before the priority date of the applicant's claim;
d). Section 25(1) (f): Not Patentable Subject Matter
That the subject of any claim of the complete specification is not an
invention within the meaning of this Act, or is not patentable under this Act;
e) Section 25(1) (g): Insufficient disclosure
That the complete specification does not sufficiently and clearly describe
the invention or the method by which it is to be performed;
ARGUMENTS ON MERITS AND HEARING SUBMISSIONS
Now here I will discuss the argument of opponent I & opponent II & its
rebuttal by the Applicant on the opposition grounds jointly.
GROUND 1: CLAIMS NOT PATENTABLE UNDER SECTION 3(c)
Opponent I arguments & hearing submission
The opponent 1 contends that the claims drawn to the polymorph 1 are not
patentable under Section 3(c) of the Act. It is an admitted fact that the
specification admits that Sorafenib tosylate is an own substance disclosed by
the WO 00/42012, WO 03/068228 and WO 03/47579. It is admitted in the
specification that a polymorphic form is prepared in WO 00/42012. No
attempt has been made by the Applicant to distinguish the properties of the
polymorphic form disclosed in WO 00/42012. In the absence of such data and
information, it must be presumed that the polymorph 1 claimed in the present
application is the same polymorph as disclosed in WO 00/42012 and
therefore, the claimed polymorph is known. Since no claim can be made to a
known substance, or properties of such known substance, the claims are liable
to be rejected. In the alternative and without prejudice, it is submitted that the
polymorphic form claimed in the present application is nothing but
characterization of the properties of the polymorph already disclosed in WO
Opponent II arguments & hearing submission
The opponent II has not pressed this ground
Applicants Argument & hearing submission to Opponent I
With regard to Section 3(c) of the Patents Act, it is submitted that the contents
of paragraph 1A of the present representation are misleading, lack proper
interpretation of law, science and are based on assumptions · and
presumptions and therefore, are vehemently denied. Opponent is wrong when
he says that the present claims 1 to 18 are drawn to polymorphic forms
of 4-{4-[( {[( 4-chloro-3-trifluoromethyl) phenyl] amino} carbonyl) amino]
phenoxy} –N-methylpyridine-2-carboxamide which is sorafenib. The correct
statement would be that the present claims relate to the polymorphic forms of
the tosylate salt of sorafenib which is different from sorafenib itself, in
particular when polymorphic forms are discussed. WO 00/42012 (referred to
as Annexure B in the Opponent's statement) does not describe any
polymorphic form of sorafenib tosylate salt. WO 03/047579 (referred to, as
Annexure Din the Opponent's statement) and WO 03/068228 (referred to as
Annexure C in the Opponent's statement) describe a tosylate salt of sorafenib
but without any specification of the polymorphic form. The polymorphic form
I of the tosylate salt of sorafenib as claimed in the present application is not
disclosed in any of the cited documents by the Opponent. It is pertinent to
mention here that the submissions made by the Opponent are based on
conjectures and surmises in as much as the Opponent has not placed on record
any evidence/ document whatsoever, which establishes the possibility of
polymorphism, is sorafenib tosylate. There has been human intervention in the
inventing subject matter of the present invention. Therefore, the subject matter
claimed by any stretch of imagination cannot be considered a mere discovery
of a scientific principle as alleged or at all and therefore, provision of Section
3(c) of the Patents Act is not attracted in the present case in hand.
GROUND 2 OPPOSTION UNDER SECTION 25 (1) (g)
THAT THE COMPLETE SPECIFICATION DOES NOT
SUFFICIENTLY AND CLEARLY DESCRIBE THE INVENTION OR
THE METHOD BY WHICH IT IS TO BE PERFORMED
Opponent I arguments & hearing submission
Though the opponent had pressed this ground in pre-grant opposition but has
withdrawn this ground during hearing.
Opponents II arguments & & hearing submission
The opponent submitted, that the whole objective of a patent grant is that a
quid pro quo system is followed, whereby the Patent Office grants a patent to
an inventor when he discloses the mode and method of performing an
invention, along with details pertaining to the invention such as prior art,
description etc. The very basis of granting a patent is to provide monopoly
right to the inventor/applicant in lieu of the disclosure of the working of the
invention to enable an unimaginative individual having sufficient skill in the
art, to perform the invention in its best embodiment.
Application describes that polymorph-II is the starting material for the
preparation of polymorph-I of Sorafenib tosylate. In this regard, impugned
application provides five examples for the preparation of polymorph-I starting
from polymorph-II.
However, as stated earlier which is not repeated here for the sake of brevity
that there is no best method disclosed for the preparation of polymorph-II. As
explained earlier, reworking of example 1 or general standard method does not
lead to the formation of polymorph-II. This leads to an unsolvable problem to
the skilled person in the field. In fact, general standard methods known in the
art do not lead to the formation of polymorph-II of Sorafenib tosylate.
Opponent highlighted during the hearing that when there is an opposition filed
by the instant opponent, Applicant mentioned various theories/points or
measures to consider while preparing polymorph-II e.g. special measures,
unintentional seeding, disappearing polymorphs, uncontaminated
laboratories etc. However, there is no whisper of such special measures,
unintentional seeding, disappearing polymorphs, uncontaminated laboratories
etc. in the specifications of the impugned application.
Applicants arguments & submission to opponent II
The Applicant would like to draw the attention of the learned Controller to the
relevant provision of the Patents Act which governs the field, i.e. Section
10(4) of the Patent Act which is reproduced herein below:
10. Contents of specifications.-
. (4) Even; complete specification shall-
(a) fully and particularly describe the invention and its operation or use and
the method by which it is to be performed;
(b) disclose the best method of performing the invention which is known to the
applicant and for which he is entitled to claim protection; and
(c) end with a claim or claims defining the scope of the invention for which
protection is claimed; (d) be accompanied by an abstract to provide technical
information on the invention:
It is submitted that the Applicant is under a mandate to comply with Section
10(4) of the Patents Act in so far as its complete specification of the patent
application is concerned. It is submitted that since the Applicant has followed
the requirements as mandated by Section 10(4) of the Patents Act, the
Opponent's objection on this ground is baseless and should be rejected
outrightly.
It is submitted that the said ground has been taken in a mechanical manner by
the Opponent without reference to the facts at hand. It must be kept in mind
that over 40 patents have been granted on corresponding applications filed in
various countries where the complete specification fulfilled the test of
describing the invention sufficiently and the best mode of carrying out the
invention.
It is submitted that the complete specification filed by the Applicant
sufficiently describes the invention as claimed in the claims of the patent
application. It is submitted that the Opponent has failed to provide any
particular detail which a person skilled in art should know to work the
invention, and which is not so disclosed. Therefore, in absence of any such
particular information, the averments are mere ipse dixit and are liable to be
ignored by the learned Controller. However, the Applicant would like to draw
the attention of the learned Controller to the fact that the polymorphic forms
of sorafenib tosylate are sufficiently and clearly described in the description in
tables 2 to 6 by X-ray diffractometry, IR spectroscopy, Raman spectroscopy,
FIR spectroscopy and NIR spectroscopy.
It is submitted that the complete specification filed by the Applicant
sufficiently describes the invention as claimed in the claims of the alleged
patent application.
It is submitted that the polymorph of example 1, i.e., polymorph ll, is not part
of the invention claimed in the patent application. If the whole preparation
process is conducted according to the description which is the process of
example 1 combined with the process of example 2, polymorph I is yielded
and the Opponent has confirmed this. The Opponent is trying to approbate and
reprobate at the same time which cannot be allowed by the learned Controller.
Therefore, the disclosed process provides the claimed subject matter
(polymorph I) sufficiently and there is no lack of enablement as alleged or at
In view of the above, it is submitted that the complete specification of the
patent application sufficiently and clearly describes the invention as well as
the method by which it is to be performed. Therefore, the Opponent's
objection on this ground as well should be rejected outrightly.
In this regard, the Applicant also refers to and relies upon oppositions filed by
the parent Company of the Opponent and Biofer S.P.A. before the EPO
against corresponding European patent. (Reference in this regard is made to
the internal pages 4 and 5 of the communication dated October 31, 2013 by
Attorneys for the Patentee before the EPO and internal page 6 and pages 7 and
8 of the communication dated May 28, 2014 of the EPO and internal pages 2
and 3 of the communication dated December 5, 2014 by Attorneys for the
Patentee before the EPO).
GROUND 3: CLAIMS NOT PATENTABLE UNDER SECTION 3(i)
In view of deletion of claims 5 to 6 and 9 to 11, which were related to the use
of the compound of claim 1 or a pharmaceutical composition thereof for the
treatment of certain disorders or diseases during reply to First Examination
Report dated 04th March; 2014.The opposition ground is rendered moot.
GROUND 4: CLAIMS NOT PATENTABLE UNDER SECTION 3(e)
Opponent I arguments & hearing submission
The opponent 1 submitted that claims of the impugned application are drawn to polymorphic form 1 as well as the composition comprising such form as well as combinations containing such form I. However, the specification does not provide any examples for preparation of such composition nor are there any examples to demonstrate that the compositions claimed are
synergistic. Therefore, the composition is no more than a mere admixture and is liable to be rejected.
Applicant's arguments & hearing submission to applicant I
The applicant submitted that the Opponent is trying to mislead the learned Controller with wrong interpretation of the claims of the Patent Application. It is submitted that as long as the polymorphic form I of compound of formula I itself is patentable, any composition comprising said polymorphic form I ought to be patentable as well and as such provision of Section 3(e) are not attracted in the present case in hand.
Opponent II arguments & & hearing submission
The opponent 2 asserts that claims 5-7 claimed in the specification of the
impugned application fail to provide any data to support the statement that
these pharmaceutical compositions or combinations provide any synergistic
effect. In the absence of such data, the compositions or combinations in these
claims are a mere admixture of the known substances. Hence, such claims
ought to be rejected solely on the basis of this ground.
Applicants arguments & hearing submission to Opponent II
The applicant submitted that the Opponent is trying to mislead the learned Controller with wrong interpretation of revised claims 5, 6, 7, 10 to 13 on record (which correspond to earlier claims 7, 8, 12, 15-18) of the patent application. It is submitted that as long as the polymorphic form I of compound of formula I itself is patentable, any composition comprising said polymorphic form I ought to be patentable as well and as such the provision of Section 3(e) is not attracted in the present case in hand.
GROUND 5- CLAIMS NOT PATENTABLE AS LACKING
NOVELTY UNDER SECTION 25(1) (b)/(c)
Opponent I arguments & & hearing submission
The opponent contends that the patent application itself discloses and admits
that Sorafenib tosylate salt is disclosed by WO 00/42012, WO 03/47579 and
WO 03/068228. Scott Wilhelm et al narrate the use of the compound BA43-
9006 in clinical settings- the same constitutes a specific use of the compound
BA43-9006. The said compound would have existed in its most stable form
i.e. Form 1. In the absence of any data to the contrary, it must be presumed
that the above documents disclosed the polymorph 1 as claimed in the
impugned application and hence, polymorph 1 stands anticipated by aforesaid
Applicants' arguments & hearing submission to Opponent I
The applicant submitted that the present claims relate to the polymorphic form
I of the tosylate salt of sorafenib. WO 00/42012 does not describe any
polymorphic form of sorafenib tosylate salt. WO 03/047579 and WO
03/068228 describe a tosylate salt of sorafenib but without any specification
of the polymorphic form. The polymorphic form I of the tosylate salt of
sorafenib as claimed in the present application is not disclosed in any of the
documents cited by Opponent. Therefore, the subject matter claimed is novel.
The applicant also submitted that the patent application discloses an invention
which pilots to higher stability and better physical characteristics of Sorafenib
in the form of polymorph I of tosylate salt. It is important to distinguish the
present invention from the disclosure in the prior art document which pertains
to Sorafenib to cure cancer.
It is submitted that none of the cited documents read alone or in combination
disclose any polymorph of sorafenib tosylate, let alone polymorphic form I,
any pharmaceutical composition thereof or any process for making the said
polymorphic form. A person skilled in the· art cannot predict the
polymorphism and prepare the subject compound from the available
disclosure therein. Therefore, polymorphic form I of sorafenib tosylate is not
anticipated in view of the cited documents.
Opponent II arguments & hearing submission
Claim 1 of the impugned application relates to polymorph-I of Sorafenib
tosylate (in general referred to as compound of claim 1).
Applicant states that polymorph-I can be prepared by converting polymorph-II
of Sorafenib tosylate. Applicant states that polymorph-II is obtained while
converting Sorafenib free base to Sorafenib tosylate via following any general
standard method for the preparation of tosylate salts or by following the
procedure given in example 1 of the impugned application (see page 2, first
paragraph and example 1 of the impugned application).
As explained in the representation, Opponent repeated the said example 1 and
observed that polymorph-I was obtained in contrary to the Applicant's
statement. To ensure the reproducibility example 1 was repeated four times;
however in all the experiments Polymorph-I was obtained (refer to
chromatographs submitted with the representation).
To follow the Applicant's statements, Opponent also followed a general
standard method for the preparation of Sorafenib tosylate. In this regard,
Opponent prepared the tosylate salt of Sorafenib by following an alternative
method known in the art that is found in document D2 (see page 11, example
3).The said example 3 of D2 was re-worked replacing the thiazolidine
compound by Sorafenib, and again polymorph-I of Sorafenib tosylate was
obtained (see chromatographs submitted with the representation).
From above, it is clear that by following any method for the preparation of
Sorafenib tosylate from Sorafenib, polymorph-I is obtainable. Accordingly, it
was submitted that D1 which is an admitted prior art and which discloses
Sorafenib tosylate is nothing other than polymorph-I.
Therefore, it was submitted that the subject matter of claim 1 of the impugned
application is not new. Hence, D1 (which is an admitted prior art) disclosing
Sorafenib tosylate inherently anticipates subject matter of claim 1.
Also, reference to the counterpart of the instant application in Europe under
opposition was made by the Opponent. Wherein, counterpart of the impugned
application is under opposition filed by the two opponents'. Among two of the
opponents, one is the parent company of the instant Opponent and second
named as Biofer SpA. It was observed and submitted before the Ld. Controller
that Biofer SpA also arrived at polymorph-I of Sorafenib tosylate by following
general standard method OR example 1 of the impugned application and
polymorph-II is not obtainable by methods described in the impugned
In this regard, Applicant submitted that such phenomenon is caused by an
unintentional seeding or disappearing polymorph theory (see page 18, line
07 of "Statement and Evidence").
Again, Applicant states that polymorph II can only be produced when taking
special measures or might only be reproduced in uncontaminated
laboratories (see page 18, line 15 onwards of "Statement and Evidence").
It was submitted by the Opponent that there is no whisper of such special
measures, unintentional seeding, disappearing polymorphs,
uncontaminated laboratories etc. in the specifications of the impugned
Opponent submitted that there is no need to take special measures while
following a prior art. Accordingly, if by following a prior art someone arrives
at the subject matter of the instant application then it should be considered as
novelty destroying prior art.
In this regard, Applicant relied upon following case law for support
which was handed-out during the hearing. The relevant paragraphs there
from are set-out for ready reference:
SMITHKLINE BEECHAM CORPORATION and BEECHAM
GROUP, P.L.C., V. APOTEX CORP., APOTEX, INC., and TORPHARM,
Factual background of the case:
In 1970s, a British company, Ferrosan, invented a compound known as
paroxetine, See U.S. Patent No. 4,007,196 ('196 patent). Ferrosan eventually
developed a process to produce the crystalline hydrochloride salt of
paroxetine, or paroxetine hydrochloride (PHC).
In 1980, Ferrosan licensed the '196 patent and its other PHC-related
technology to SmithKline. SmithKline began manufacturing PHC in its
Harlow plant in England.
In 1985, Alan Curzons, a chemist in SmithKline's Worthing, England
laboratory discovered a new crystalline form of PHC while attempting to
improve PHC production.
"Curzons's test results established that the new product was the
hemihydrous form of PHC (PHC hemihydrate). Ferrosan's original form
was anhydrous PHC (PHC anhydrate)."
"In 1985, SmithKline filed a patent application in the British Patent Office
relating to "crystalline paroxetine hydrochloride, its preparation and its uses
as a therapeutic agent." The British application identified the invention as
both the hemihydrate and the anhydrate form of PHC, as well as mixtures that
contain a major portion of either form.
One year later, on October 23, 1986, SmithKline filed a U.S. application
claiming priority to the British application that issued as the '723 patent in
1988. The '723 patent does not claim PHC anhydrate and does not claim
mixtures of the two PHC forms. The only claim at issue in this case is claim 1,
which reads in its entirety: "Crystalline paroxetine hydrochloride
hemihydrate."
In 1998, Aptex while seeking approval to market its own PHC identified the
active ingredient in its antidepressant as PHC anhydrate.
In 1998, SmithKline asserts that Apotex will infringe by manufacturing PHC
anhydrate tablets that necessarily contain, by a conversion process discussed
below, at least trace amounts of PHC hemihydrate.
Page 05, 2nd para:
"To show that manufacture of PHC anhydrate tablets necessarily creates PHC
hemihydrates: SmithKline proffered expert testimony on the so-called
"seeding" or "disappearing polymorph" theory. Under this theory, Ferrosan
may have originally created a crystalline compound, namely PHC anhydrate,
in a relatively unstable form. For presently unknown reasons, the PHC
anhydrate "morphed" into a more stable form, namely the PHC hemihydrate
discovered in SmithKline's facilities. With this new form or polymorph in
existence, SmithKline's experts explained, the general environment became
"seeded" with crystals of PHC hemihydrate. In this seeded environment, the
PHC anhydrate converts to the PHC hemihydrate upon its inevitable contact
with seeds of PHC hemihydrate. In other words, the creation of pure PHC
anhydrate became extremely difficult, if not impossible; the old polymorph,
PCH anhydrate, has effectively disappeared in its pure form because it
changes naturally into the new polymorph, PCH hemihydrate."
Page 16, 2nd para
"SmithKline argues that practicing the '196 patent infringes claim 1 of the
'723 patent, but that the '196 patent does not anticipate claim 1 of the '723
patent. SmithKline uses the "disappearing polymorph" theory to justify its
apparently inconsistent positions. On the one hand, SmithKline asserts that
the creation of a prior art compound will result in a product containing at
least trace amounts of their patented compound. On the other hand,
SmithKline contends that the creation of the prior art compound before
SmithKline's discovery of its compound did not have the same result."
Page 18, last para:
The '196 patent is undisputed prior art under 35 U.S.C. § 102(b), even though
the '196 patent discloses how to make PHC anhydrate and does not discuss
PHC hemihydrate. PHC hemihydrate was not even discovered until years after
the '196 patent was filed. Nonetheless, the '196 patent anticipates claim 1 of
the '723 patent because the '196 inherently discloses PHC hemihydrate.
Page 20, line 06 onwards:
Apotex did not need to prove that it was impossible to make PHC anhydrate in
the United States that contained no PHC hemihydrate, but merely that "the
disclosure [of the prior art] is sufficient to show that the natural result flowing
from the operation as taught [in the prior art] would result in" the claimed
product. In re Oelrich, 666 F.2d 578, 581 (CCPA 1981); accord
Mehl/Biophile Int'l Corp., 192 F.3d at 1366; see also Atlas Powder, 190 F.3d
at 1349-50 (affirming the district court's finding of inherent anticipation
despite a finding that the inherent element could be avoided by taking
"extraordinary measures" when practicing the prior art).
Page 21, first line:
723 held invalid for anticipation by the 196 patent.
SmithKline did not offer any evidence that pure PHC anhydrate can be
produced in facilities that are not seeded.
This court's law does not require Apotex to take extraordinary measures to
practice the prior art without infringing claim 1 of the '723 patent. See Atlas
Powder, 190 F.3d at 1349-50 (affirming the district court's finding of inherent
anticipation despite a finding that the inherent element could be avoided by
taking "extraordinary measures" when practicing the prior art).
Therefore, drawing the analogy with the instant case, if so called
disappearing polymorph theory exists, then eventually at some stage
before the filling of instant application Polymorph-I must have formed.
Also, court in the above cited case made clear that there is no need to take
special measures while following a prior art. Therefore, it was submitted
that subject matter of the instant claim cannot be considered novel on
view of D1.
SUBMISSIONS IN RELATION TO NOVELTY [U/S 25(1) (b)]
Opponent submitted that it is evident from D3 that Modification-3 of
Sorafenib tosylate was used in the clinical studies. Again, D3 makes it clear
that a tablet formulation of Sorafenib tosylate was used in the clinical studies.
Also, from D4 it is evident that clinical studies were conducted before the
priority date of the impugned application.
In this regard, it was submitted that it's a common general knowledge that
only a stable form of a compound can be used in the formulation of a
composition. Applicant admitted the same that "compounds in most stable
form are required for efficacious and stable drug formulation" (refer to
Page 12, para 21, line 05-06 of the "Statement and Evidence").
Therefore, it was submitted that any composition of Sorafenib tosylate would
certainly use stable form which is as per Applicant's statement is polymorph I.
Accordingly, D1 (see page 60, line 04 -08 of D1) which is Applicant's own
international application and which discloses the tablet compositions of
Sorafenib tosylate would certainly use the stable form for the efficacious and
stable drug formulation. Therefore, subject matter of the claim 1 lacks novelty
Claims 5-7 and 10-13
D1, inter alia discloses the subject matter of claims 5-7 and 10-13 as presented
in table 2 of the representation which is not repeated herein for the sake of
Applicant's arguments & hearing submission to Opponent II
It is submitted that grounds of opposition under Section 25(1)(e) of the Patents
Act does not relate to novelty/anticipation as has been the understanding of
the Opponent, but to inventive step. The Opponent has wrongly relied upon
Section 25(1) (e) to challenge the novelty of claim 1.
lt is submitted that Example 1 according to the patent application describes
the preparation process of the metastable polymorph II of sorafenib tosylate.
However, the present invention is about the stable polymorph I of sorafenib
tosylate. Therefore, example 1is not an example to support the claims but is to
demonstrate which polymorphic form was obtained at the time the
invention was made, i.e., prior to the filing date, when conducting the process
as described in examples. The figures and tables of the patent application
show the analytical data obtained for said polymorph ll. Therefore, evidence is
provided for the existence of polymorph II.
It is submitted that the Opponent now asserts that they could not reproduce
polymorph II when conducting the process described in example 1 as per the
declaration. It is submitted that while the said declarations have no
recognition under the law and are inadmissible as evidence for the reasons as
stated in the preliminary paragraph, the process of example 1 was not carried
in a proper manner. Accordingly, this could have happened for the following
reason. It is known that established methods may sometimes result in a
different crystalline product than usually expected, although all process
parameters and starting materials have remained identical. Such phenomenon
is caused by an "unintentional seeding (‘‘Disappearing Polymorphs", Ace.
Chem. Res., voL 28, 1995, 193-200 which is annexed with the statement
Annexure A). In the field of the art, this phenomenon is explained by minimal
concentrations of a certain crystalline modification that serves as a seeding
nucleus and thus, prevents the development of another crystalline form that
had always been obtained up to then. Consequently, this phenomenon can be
ascribed to a contamination of the laboratories and lab equipment. It is clear
that particularly in the laboratories of the Opponent, polymorph I was already
handled before the reproduction of Example 1, causing the results obtained
by the Opponent. However, this does not mean that polymorph II can never
be produced by the method according to Example 1, but that Example 1
might only be reproduced in uncontaminated laboratories (i.e. in laboratories
different from those of the Opponent).
With regard to the submissions made by the Opponent re Dl (WO
3/068228), it is submitted that it merely mentions a tosylate salt of sorafenib
by name but without any further disclosure, i.e., it neither describes a method
for its preparation nor provides a specification of the any polymorphic form.
That means none of the now identified polymorphic forms I, II and II were
disclosed in Dl and this is true in particular for the polymorphic fom1 I of the
tosylate salt of sorafenib as claimed in the patent application. Prior to the
present invention of polymorph 1, a standard procedure was performed by the
Applicant for the preparation of sorafenib tosylate which had yielded
polymorph II as described in example 1of the patent application. One of the
reasons why the Opponent could not have reproduced polymorph II is
explained above. The only information the public domain had at the filing
date of the patent application was that sorafenib tosylate exists, but without
any information about the polymorphic forms. Therefore, D1 cannot destroy
the novelty of claim 1or any other claims of the patent application on record as
alleged or at all.
With regard to the submissions made by the Opponent re 02, it is submitted
that example 3 of D2 cannot anticipate the subject matter of the patent
application, since the example is not directed to the preparation of "sorafenib"
tosylate and 02 discloses a crystalline tosylate salt and a method for its
preparation, which does not contain any reference of the compound
"sorafenib". Therefore, D2 cannot destroy the novelty of claim 1 or any other
claims of the patent application on record as alleged or at all. (Reference in
this regard is made to the pages 17 to 19 paragraphs 33 to 37 of the statement
in support of the application)
CLAIMS 5 TO 12- LACK OF NOVELTY [SECTION 25(1) (b)
It is submitted that claims 5, 6, 9, 10 and 11 referred to by the Opponent have
already been deleted while filing response to the First Examination Report
dated March 4, 2013. Therefore, the assertions by the Opponent in paragraph
under reply in relation to the claims 5, 6, 9, 10 and 11do not survive anymore.
In so far as the submissions made by the Opponent re claims 7, 8 and 12
(renumbered as claims 5, 6 and 7 respectively) are concerned, it is submitted
that for the reasons as stated in the preceding paragraphs, since polymorph I
of sorafenib tosylate is novel, claims 7, 8 and 12 (renumbered as claims 5, 6
and 7 respectively) are novel as well. In view of the above, it is submitted that
the submissions made by the Opponent in paragraph under reply are baseless
and ought not to be taken cognizance of by the learned Controller. (Reference
in this regard is made to the pages 22 to 23 paragraphs 45 and 46 of the
statement in support of the application).
In this regard, the Applicant also refers to and relies upon oppositions filed by
the parent Company of the Opponent and Biofer S. P.A. before the EPO
against the corresponding European patent The communication dated
October 31, 2013 by Attorneys for the Patentee before the EPO, the
communication dated May 28, 2014 of the EPO, the communication dated
December 5, 2014 by Attorneys for the Patentee before the EPO along with
the documents/evidence filed in support of its submissions made therein are
annexed hereto and marked as Annexure 11 (colly). (Reference in this regard
is made to the internal pages 10 and 11 of the communication dated Octobe31,
2013 by Attorneys for the Patentee before the EPO and internal page 8
paragraph12.4 of the communication dated May 28, 2014 of the EPO).
GROUND 6: PRIOR USE SECTION 25(1) (d)
Opponent I arguments & hearing submission
It is submitted that sorafenib tosylate is well known and widely used before
the date of the impugned application and hence the polymorph as claimed by
the impugned specification is known from prior art disclosures. It is further
submitted that sorafenib tosylate (in the form I as claimed in the impugned
application) has been subject of extensive prior use by the Applicant. Such
prior use has been well documented as in Current Pharmaceutical Design
2002, 8, 2255-57 titled 'Bay 43-9006: Preclinical Data' by Scott Wilhelm et al.
The said article narrates the use of the compound Bay43-9006 in pre-clinical
settings (Annexure E). The compound would exist and would have been used
naturally in its most stable form i.e. form I. Such documents constitute prior
use of the polymorphic compound claimed.
Thus, all claims stand anticipated by disclosure in prior art, prior claiming and
by prior use and hence this application, ought to be rejected.
Applicant's arguments & hearing submission to Opponent I
It is submitted that the Opponent wrongly asserts that the polymorphic form I
of sorafenib tosylate is known from prior art disclosures. It is correct that
sorafenib tosylate is mentioned in WO 03/047579 and WO 03/068228 but
without any specification of the polymorphic form. The Opponent does not
provide evidence of any prior use of the polymorphic form I of sorafenib
tosylate. The document Scott Wilhelm.et al. "Current Pharmaceutical Design
2002" [referred to as Annexure E in the Opponent's statement] only describes
the use of the compound BAY 43-9006 which is sorafenib itself but it is not
even the tosylate of sorafenib, let alone arty polymorphic form thereof. It is
submitted that none of the prior mentions the use any polymorphic form of
sorafenib tosylate, which is the subject matter of the present invention.
Therefore, no evidence is provided by the opponent for prior use of the
polymorphic form I of sorafenib tosylate.
The contention of the Opponent that the present invention stands anticipated
by prior use is a bald averment in the absence of any evidence to the contrary,
and therefore, this ground of the Opponent is liable to be rejected.
Opponent II arguments & hearing submission to applicant
Compound of Claim 1 i.e. polymorph-1 of Sorafenib tosylate was well known
and widely used before the priority date of the impugned application.
D3 and D4 are post published documents; however, it is a well set rule that
publication is not necessary to establish prior knowledge or prior use. A
matter may be publicly known even if unpublished, if for instance, it is
Hence, these documents can be used as proof of evidence to establish prior use
or prior knowledge of polymorph-I of Sorafenib tosylate.
D3 describes the use of the compound of claim 1 before the priority date of
the impugned application. D3 on page 3/49 under the heading, "Active
Substance" describes that, "The active substance exhibits polymorphism and it
crystallizes in three different modifications (Mod I, Mod II and Mod III). "
Under the heading, "Manufacture", it states that, "The active substance is
visually tested for appearance and its identity is confirmed by NIR and HPLC,
and the desired modification of Sorafenib tosylate (Mod I) is confirmed by
Raman spectroscopy. Also, it discloses that (on page 4/49), "The potential for
polymorphism was investigated by Raman spectroscopy and found to be
unchanged"
D3, on page 28/49 under the heading, "Main studies", it relates to the studies
100391 and 11213 performed during Phase II and Phase III trials. Also, it has
been acknowledged on page 30/49 under the heading, "Blinding (masking)"
that, "The active and placebo tablets were identical in appearance".
Hence, from the above, it is clear that polymorph-I of Sorafenib tosylate was
used in phase II and phase III clinical studies.
From D4, it is evident that the clinical studies 100391 and 11213 for Sorafenib
tosylate were started on September 25, 2002 and November 15, 2003 (Page 3,
Point 5) respectively, i.e. before the priority date of the impugned application.
It is also known that the product which is ultimately approved is necessarily
the one which has been clinically tested, this being a strict requirement of drug
approving agencies such as the FDA or EMA.
Hence, it is concluded that, during phase-II and Phase-III studies polymorph-I
of Sorafenib tosylate was handed out to the patients prior to the priority date
of the impugned application.
In Bilcare Limited v. Amartara (P) Ltd. (lA Nos. 10848/2006, 1397112006
and 11160/2006 in CSOS No.l847/2006), "Prior public knowledge of the
alleged invention which would disqualify the grant of patent can be by word of
mouth or by publication through books or other media. If the public once
become possessed of an invention, says Hindmarch on Patents, by any means
whatsoever, no subsequent patent for it can be granted either to the true or
first inventor himself or any other person, for the public cannot be deprived of
the right to use the invention . the public already possessing everything that
he could give" [Source www.judis.nic. in].
As shown above, the subject matter of impugned application was in
possession of the public before the priority date of the impugned application,
hence lacks novelty VIS 25(1) (d), and hence, ought to be rejected on this
Applicants argument & hearing submission to Opponent II
It is submitted that the Opponent cites two clinical studies 100391 and 11213
which had started prior to the filing date. However, the Opponent does not
provide any information on both studies which were published prior to the
filing date. The later published EMEA document refers back to both studies
but this information was not available prior to the filing date. The Opponent
asserts that both studies cause a public prior use of polymorph I of sorafenib
tosylate. This is not shown by dear I cogent evidence. The clinical studies
were not conducted in the public domain but were subject to a specific
confidentiality. In other words, neither the physicians, nor the clinical staff,
nor the patients were allowed to give the tablets used in the study into the
public. The tablets had to be used only in connection with the clinical study,
which was confidential Therefore; no tablet went into the public domain. It is
submitted that the Opponent failed to give clear I cogent evidence re how I to
whom/ where and when a tablet had been provided to the public prior to the
Without prejudice to what has been stated above, it is submitted that D3
relates to a scientific discussion of the pharmacological activity of sorafenib in
treating cancer, published by the EMEA in 2006, i.e. after the filing date of the
present patent application. D3 reports on two clinical studies 100391 (phase II
study) and 11213 (phase III study), allegedly using film-coated tablets
containing 274 mg of sorafenib tosylate, microcrystalline
cellulose,croscarmellose, hypromellose sodium lauryl sulphate, magnesium
stearate, water, titanium dioxide and red ferric oxide (03, page 2149, last
D3 mentions that the active substance exhibits polymorphism and crystallizes
in three different modifications (modification t It and III) (D3, page 3 j 49,
first paragraph). However, D3 does not give any hint as to which of the
modifications have been used for the clinical studies (page 29/49, second
paragraph and page 36/ 49).
The Opponent further refers to document D4 (approval letter by FDA). From
D4, it arises that the studies were carried out between September 25, 2002 and
September 2006 (study 100391) and between November 19, 2003 and
September 2006 (study 11213) (see e.g. 04, page 3, item 5).
D4 mentions the date when the clinical studies started. However, the date of
delivery of the sorafenib tosylate tablets, i.e. the actual "use", does not
necessarily correspond to the date of the start of the study. A "use" of the
subject matter of the invention before the priority date of the present patent
application (September 29, 2004) has not been evidenced.
It is submitted that the Opponent does not provide any information to prove
that both the studies were published and were in public domain prior to the
filing date of the present application. The later published EMEA document
(D3) refers back to both the studies but this information was not available
prior to the filing date of the present application. Additionally, neither D3 nor
D4 evidences that sorafenib tosylate in the form of polymorphic form I has
been used in the clinical trials. It is also submitted that the clinical studies
were not conducted in the public domain, but were subject to a specific
confidentiality. The medical investigators were obliged to keep any
information as to the study strictly confidential
Without prejudice to what has been stated hereinabove, it is submitted that for
the purpose of Section 25(1)(d), the invention so far as claimed in any claim
of the complete specification should have been publicly known or publicly
used in India before the priority date of the claim. In view of the fact that the
Opponent has not provided any evidence cogent or otherwise, as to the prior
use of the invention disclosed in the patent application before the priority date
of the claim, the present ground is baseless and has no merit and accordingly,
ought not to be taken cognizance of by the learned Controller.(Reference in
this regard is made to the pages 19 to 22 paragraphs 38 and 44 of the
statement in support of the application).
It is submitted that it is a settled proposition of law that the onus was on the
applicant to show that the prior use was known and not secret. Further, if
published information does no more than disclose the existence of product
which is not physically obtainable by the public, that product cannot have
been said to have been "made available" unless and until the public has been
told how it can be produced. In this regard the Applicant refers to and relies
upon the case titled Air Master Equipments India (P) Ltd v Ramesh Nana
Mhatre (2011) SCC online IPAB 31 at paragraph 31 which is annexed hereto
and marked as Annexure·12.
At this stage, it is once again pertinent to draw the attention of the learned
Controller to the oppositions filed by the parent Company of the Opponent
and Biofer S.P.A. before the EPO against corresponding European patent
wherein the Applicant had placed evidence on record by way of an affidavit of
Ms Jeanne Lewis wherein she confirmed that all materials used for staff
training were marked as "confidential", assuring the content to be the
Applicant's property. (Reference in this regard is made to the internal pages 6
to 10 of the communication dated October 31, 2013 by Attorneys for the
Patentee before the EPO, internal page 6 and pages 8 and 9 of the
communication dated May 28, 2014 of the EPO and internal pages 3 to 6 of
the communication dated December 5, 2014 by Attorneys for the Patentee
before the EPO).
GROUND 7: CLAIMS LACK INVENTIVE STEP UNDER SECTION
25(1)(e)
Opponent I arguments & hearing submission
The opponent has relied on the documents
The impugned application admits at WO 00/42012 that one form of the
polymorph is already disclosed. "The compound of the formula {II} is
prepared in the manner described in WO 00/42012. The compound of the
formula {I} is prepared according to a general standard method for the
preparation of tosylate salts, as described in example I of the working
examples. In this method, the compound of the formula {I) is obtained in one
crystal polymorph which is referred to herein below as polymorph II.
Polymorph II has a transition point of 194°C and a characteristic X-ray
diffractogram, IR spectrum, Raman spectrum, FIR spectrum and NIR
spectrum (Tab. 1-6, Fig. 1- 6}. It has been found that polymorph II is
metastable." (See para 1 of page 3 of the impugned specification)
Assuming but not conceding that the impugned application pertains to the
conversion of one polymorphic form to another polymorphic form, it is
submitted that the conversion of one crystalline form to another crystalline
form is known from various prior arts which are in public domain since a long
time and much before the priority date of impugned patent application.
For instance W0/1996/027592(attached herewith as Annexure F ) published
on 12 September 1996 teaches process for the preparation of polymorphic B
form of (E)-4-[[3-[2-(4-cyclobutyl-2-thiazolyl)ethenyl]phenyl]amino]-2,2-
diethyl-4-oxobutanoic acid by agitating Polymorphic Form A in a solvent and
adding the seed crystals of Form B. Also, a disclosure in W0/1999/001444
(attached herewith as Annexure G) published on 14 January 1999 (Example
23) teaches process for the preparation of thermodynamically stable
polymorphic form I of the tachykinin receptor antagonist 2-(r)-(1-(r) -(3,5-
bis(trifluoromethyl) phenyl)ethoxy)-3-(s)-(4-fluoro) phenyl-4-(3-5 (-oxo-
1hAh-1,2A,-triazolo) methylmorpholine by effecting the compound of Form II
in methanol and seeded with crystals of Polymorphic form I.
Thus, the conversion of one polymorphic form of compound to another is
spontaneous and is also known from prior art and is within the routine skills of
a person skilled in the art. Moreover, the use of inert solvent and seeding
technology, which appears to be the crux of claims 2-4 of impugned
application, is also in the public domain.
Claims 2-4 and claims 13-14 of the impugned patent application deal with
process for the preparation of polymorphic form I of sorafenib tosylate. Claim
13- 14 deal with the process of converting from polymorph II to polymorph
I."The process involves effecting the polymorphic form II of sorafenib tosylate
in an inert solvent and stirring or shaking until Polymorphic Form I was
obtained".
It is submitted that stirring and shaking are but means to accelerate the
conversion and even without such process, the polymorphic form II is
automatically converted to polymorphic form I.
Polymorph-I of sorafenib tosylate is found to be the most stable crystal form
and claimed as a compound in claim-1 of the impugned application. The
pharmaceutical composition and method of use claims in the said application,
all deal with polymorph-I only as this is 'stable'.
The process and methods used for making polymorphs or converting a
metastable polymorph into a stable polymorph is well documented in
literature. For any a chapter from the book "Advanced Pharmaceutical Solids"
by Jens T. Carstensen, published by Marcel Dekker Inc, 2001""" -(see
Annexure H) clearly discloses the various experimentation techniques to
obtain polymorphs and to convert metastable polymorphs into stable
Thus from the above following are established
1. The conversion of one polymorphic form to another polymorphic form is
well known in prior art.
2. The use of the inert solvent and seeding technology for the conversion one
polymorphic form to another polymorphic form is known from the prior art.
Hence, the process of conversion of polymorphs and the resultant
polymorphic form is well known from prior art. On this ground alone all
claims ought to be rejected.
It is submitted that the claims of the impugned application are obvious in view
of what was already known in the art. The Applicant admits at WO 00/42012
that a certain polymorph of tosylate salt of Sorafenib is known. Techniques for
development of polymorphic forms were already known in the prior art.
Techniques for conversion of 1 polymorphic form to another were also known
and taught - for example WO 1996/027592 (which teaches preparation of
polymorphic B form of (E)-4-[[3-[2-(4-cyclobutyl-2 -thiazolyl) ethenyl]
phenyl] amino]-2, 2-diathyl-4-oxobutanoic acid by agitating Polymorphic
Form A in a solvent and adding the seed crystals of Form B).Similarly, WO
1999/001444 teaches a process of converting thermodynamically stable
polymorph 1 of tachykinin receptor antagonist to Form 2 by treatment in
methanol and seeding.
It is submitted that conversion of one polymorphic form of a compound to
another polymorphic form is often spontaneous and even if induced, the
conditions for such conversions were practices routinely and well within the
skills of a person skilled in the art. No inventive step is found in such
conversion. The process claims merely recite the conversion of form 2 of
Sorafenib tosylate in an inert solvent which shaking/stirring in order to obtain
Polymorphic Form 1. Such processes are very common in the prior art. Jens T.
Carstensel 2001 in the book "Advance Pharmaceutical solvents" discloses
various techniques for conversion of metastable form into stable polymorphs.
Therefore, obtaining stable polymorphic form of Sorafenib tosylate was the
result of the techniques already known in the prior art and no inventive step
resides in the same. There is no merit or force in the argument of the
Applicant that finding the most stable and superior polymorphic form is
inventive nor force in the argument that the prior art does not describe
conversion of any polymorphic form of Sorafenib tosylate. Because, even if
the prior art does not expressly teach preparation of Sorafenib tosylate
polymorphic form, the techniques for preparation thereof were well known
and therefore, Sorafenib tosylate polymorph as well as processes for its
preparation were obvious and within the routine skills of a person skilled in
the art. In view of the above, the claims are obvious and liable to be rejected
on this ground alone.
Applicant's argument & hearing submission to opponent 1
The contents of paragraph III of the statement of opposition are denied in toto and are wrong. Claims of the present Application are valid and involve inventive step.
Document WO 00/42012 does not describe the polymorphic form I of the tosylate salt of sorafenib. None of the cited documents describe any type of polymorphic form of sorafenib tosylate. The Opponent is wrong when he starts the discussion with the conversion of polymorph II of sorafenib tosylate into polymorph I. Even polymorph II was not described in the prior art. Also the documents WO 1996/027592 (referred to as Annexure F in the Opponent's statement) and WO 1999/001444 (referred to as Annexure G in the Opponent's statement) are not relevant because they do not refer to sorafenib at all and only describe the conversion of one polymorph into another polymorph of a different chemical compound. The question regarding inventive step is not a question whether the process of conversion of one polymorph into another polymorph is obvious but it is the question what are the polymorphic forms and which form is surprisingly superior over the other polymorphs. The discussion whether the conversion of one polymorph into another would be obvious is not eligible here because no polymorphic form of sorafenib tosylate is known in the prior art.
In general there are substances which only appear in a single crystal form but there are also substances which can form two, three or even more polymorphous crystal modifications. It is just as difficult to calculate or predict this possible morphological and structural variety and the respective physico-chemical, especially thermodynamic stability thereof on a scientific-mathematical basis, as it is to calculate or predict their different behaviour. The relevant polymorphism of an organo-chemical substance is always unpredictable in respect of the number of polymorphs, the stability thereof and their behaviour. Due to the unpredictability the screening for polymorphs is not a routine work. The finding of new polymorphs having improved properties does not constitute a mere discovery but fulfils all
criteria needed for an invention.
The problem to be solved by the present subject matter was to provide a form of sorafenib tosylate which has superior properties regarding manufacturing, storage and administration. It is very important for a pharmaceutical product to have always the same constant properties. Therefore, there is a need to find the most stable form of a compound because only the most stable form can ensure that all properties and characteristics regarding stability, dissolution rate, shelf life and bioavailability remain constant during manufacturing, storage and administration. The problem is solved by the polymorph I which surprisingly is the stable form among the two other polymorphs which are meta-stable. The superior properties of polymorph I over polymorph II is demonstrated in the mechanical stress test as per the affidavit of Dr. Britta Olenik in Annexure C.
Because polymorph I of sorafenib tosylate is not obvious, its preparation is not obvious as well. Therefore, it is adequately clear that the claimed subject matter is inventive over the cited prior art and this ground of Opponent is liable to be rejected.
Without prejudice to what has been stated above, it is submitted that the conclusion drawn by Opponent that the conversion of one polymorphic form to another is spontaneous, is technically flawed. It is well understood in the art that the spontaneity of a molecule is determined through change in free energy of the molecule when it transits from one thermodynamic stage to another thermodynamic stage. This free energy, in fact, depends upon many factors like intermolecular forces, types of bonds between atoms, van-der wall forces and the like which varies according to the type and orientation of the molecule in space. It cannot be generalized that the conversion of molecule from one polymorph to another polymorph form is spontaneous based on the analysis of a molecule different in structure or properties from the molecule in question.
In regard to Annexure H, it is submitted that Annexure H elaborates the principles of polymorphism and scientific theories underlying the phenomenon of polymorphism and as such, does not further the case of the
Opponent in any manner whatsoever.
Therefore, all the assertions and conclusions made by the Opponent in ground III of the statement of opposition are wrong and are liable to be rejected in toto.
The claimed subject matter involves an inventive step and is not obvious to person skilled in the art.
Opponent II arguments & hearing submission
Claims 2,3,4,8 and 9
It was submitted that D5, which reflects the common general knowledge in the
field of polymorphism, discloses that, "The thermodynamically stable
polymorph needs to be identified. If the compound is enantiotropic, there will
be two or more stable polymorphs and transition temperatures as well. These
can be identified by simple techniques, for example by stirring or shaking
excess solid with solid at different temperatures" (see D5, page 528, column
Thus in view of D5, a thermodynamically stable polymorph of a known
compound can be identified by mere stirring and shaking the said compound
at different temperatures.
Also, Applicant states that polymorph-I is obtained by shaking or stirring at a
temperature of from 500C up to the reflux temperature of the solvent (see page
15 and bridging para at page 16).
Opponent submitted that D1 (admitted prior art) which is Applicant's own
application must have prepared Sorafenib tosylate as per general standard
method or as mentioned by the Applicant in the impugned application. If
considering the Applicant's statement that Polymorph-II is obtainable by a
general standard method, it is clear that D1 discloses polymorph-II of
Sorafenib tosylate.
Therefore, it was submitted that starting from D1 (inherently disclosing
polymorph-II) conversion of a known compound to another
thermodynamically stable polymorph cannot be considered as inventive in
Additionally, D6 provides a review of strategic approaches to remove much of
uncertainty by presenting concepts and ideas in the form of flow charts to
control the crystal form (polymorph) of drug substance.
D6 specifically mentions the use of solvents such as water, methanol, ethanol,
propanal, isopropanol, acetone, acetonitrile, ethyl acetate, hexane and
mixtures, which are the same as employed in the examples of the impugned
Therefore, it was submitted that in view of above, a person skilled in the art
setting out to convert one polymorph to another polymorph would find the
teachings of D5 as highly relevant prior art. Further to this, D6 further
removes uncertainty and provides the list of selected solvents useful in the
formation of polymorphs. Thus combining the teachings of D5 and D6, a
person having ordinary skill in the art would reach the impugned process
without ingenuity of thought.
Opponent submitted that in the Draft Manual of Patent Practice and Procedure
(2008) page 34, under 3.10.7, it is explained that "The term "obvious" means
that the invention does not go beyond the normal progress of technology but
merely follows plainly or logically from the prior art, i.e. something which
does not involve the exercise of any skill or ability beyond that to be expected
of the person skilled in the art. For this purpose a person skilled in the art is
presumed to be an ordinary practitioner aware of what was general common
knowledge in the relevant art at the relevant date. In some cases the person
skilled in the art may be thought of as a group or team of persons rather than
as a single person."
The revised Manual of Patent Office Practice and procedure Version 01.11 as
modified on March 22, 2011 states on page 79 - the general principle of
"e) If an invention lies merely in verifying the previous predictions, without
substantially adding anything for technical advancement or economic
significance in the art, the inventive step is lacking."
"g) If the invention is predictable based on the available prior art, merely
requiring workshop improvement by a person skilled in the art, the inventive
step is lacking."
Therefore, in view of the given teachings of prior art, it is evident that the
impugned process does not involve the exercise of any skill or ability
beyond which is expected of the person of ordinary skill.
Thus, based on facts presented above, the subject matter of claims 2, 3, 4,
8 and 9 lacks inventiveness (under the Act) and hence ought to be
rejected.
In this regards, Applicant states that "It is difficult to predict or
calculate physic-chemical behaviour. The relevant polymorphism of an
organo-chemical substance is always unpredictable in respect of number of
polymorphs, the stability thereof and their behaviour. Due to unpredictability,
screening for polymorphs is not routine work" (running page 23, para 49 of
the Statement and Evidence).
Opponent submitted that obviousness cannot be avoided simply by showing of
some degree of unpredictability in the art so long as there was a reasonable
probability of success.
Opponent relied upon IPAB Order: OA/8/2009/PT/CH for support
which was handed-out during the hearing. The relevant paragraphs there
from are set-out for ready reference:
Page 50, First Paragraph of the Order:
Board cited, Pfizer, Inc. v. Apotex, Inc. in 20061261 Federal Circuit
decision
The Court held that "a suggestion, teaching or motivation to combine the
relevant prior art teachings to achieve the claimed invention does not have to
be found explicitly in the prior art references sought to be combined, but
rather ‘may be found in any number of sources, including common knowledge,
the prior art as a whole, or the nature of the problem itself".
The Court held that "obviousness cannot be avoided simply by showing of
some degree of unpredictability in the art so long as there was a reasonable
probability of success.
The Court held that indeed, a rule of law equating unpredictability to
patentability, applied in this case, would mean that any new salt – including
those specifically listed in the ‘909 patent itself – would be separately
patentable, simply because the formation and properties of each salt must be
verified through testing. This cannot be the proper standard "since the
expectation of success need only be reasonable, not absolute.
Applicant states that the preparation and identification of a polymorph
depend upon a plurality of parameters (see page 25, para 53 of the Statement
and Evidence). Applicant states that there are four flow charts namely
polymorphs, hydrates, solvates, amorphous. Applicant says that D6 provides
only guidelines to characterise and synthesized. Applicant states that no
information to from D1 as to how to make different forms of Sorafenib
tosylate (refer to Page 25, para 54 and 55 of Statement and Evidence).
In this regard, IPAB in order OA/8/2009/PT/CH made it clear that the
unpredictability of success cannot rule out obviousness (refer to para 38 of the
For further clarity, IPAB cited KSR v. Teleflex case (see page 39)
It stated that the "Court for seeing obviousness need not seek out precise
teaching, but it can consider the inferences and creative steps that a person of
ordinary skill in the art would employ". The Court erred in concluding that a
patent claim cannot be proved obvious merely by showing that the
combination of elements was obvious to try. When there is a design need or
market pressure to solve a problem and there are a finite number of identified,
predictable solutions, a person of ordinary skill in the art has good reason to
pursue the known options within his or her technical grasp. If this leads to the
anticipated success, it is likely the product not of innovation but of ordinary
skill and common sense.
Para 42, Line 10-18:
In KSR, the US Supreme Court held that the analysis of obviousness must be
made explicit, and the reasoning to support the conclusion of obviousness
must be articulated with rational underpinnings, the Court may have to look
at the interrelated teachings of the multiple patents, the effect of demands
known to the design community and the background knowledge possessed by a
person having ordinary skill in the art. So the determination on obviousness is
a legal one. The Court has to see a) what is the prior art b) the differences
between the prior art and the invention and c) the skill of the imaginary
ordinary man.
Further, Hon'ble Board made it clear that who is a person having
ordinary skill in the art
Last lines of the para 42 and bridging para on next page:
Who is a person having ordinary skill in the art:
"We must remember that this ordinary man has skill in this art. He is not
ignorant of its basics, nor is he ignorant of the activities in the particular field.
He is also not ignorant of the demand on this art. "He is just an average
man. Well. just an ordinary man." But he is no dullard. He has read the
prior art and knows how to proceed in the normal course of research with
what he knows of the state of the art. He does not need to by guided along
step by step.
He reads the prior arts as a whole and allows himself to be taught by what is
contained therein. He is neither picking out the" teaching towards passages"
like the challenger, nor is he seeking out the "teaching away passages" like
the defender."
Page 55 of the order:
Exhibit A speaks of using lower alkyl group for Pegylation.
Ex.B says that PEG modification has several advantages. We have already
referred to this prior art.
Ex C is referred in Monfardini as encouraging use of high molecular weight.
So these materials provided the knowledge to the person skilled in the art
regarding the advantages of branched Pegylation of IFN. He would use the
conventional methods as did the inventor. If the methods used are
conventional, there is no difficulty in the methods. Even if one grants a degree
of unpredictability in the behavior of interferon there was a greater reason to
expect success.
Therefore, drawing analogy with the present case,
D1 discloses Sorafenib tosylate
D5 describes the methodology for the identification of
thermodynamically stable form of a compound.
D6 disclosed the selected solvents mainly used in polymorphism
Therefore, in view of the given teachings of the prior art, it is evident that
the impugned process does not involve the exercise of any skill or ability
beyond which is expected of the person skilled in the art.
With regard to Applicant's concern on Hindsight, Opponent relied
upon Application of Gerald McLAUGHLIN, Patent Appeal No. 8474,
United States Court of Customs and Patent Appeals:
On page 03, para 21:
We have taken the above argument into consideration and do find that it has
some merit. Nevertheless, it is not convincing. It should be too well settled
now to require citation or discussion that the test for combining references is
not what the individual references themselves suggest but rather what the
combination of disclosures taken as a whole would suggest to one of ordinary
skill in the art. Any judgment on obviousness is in a sense necessarily a
reconstruction based upon hindsight reasoning, but so long as it takes into
account only knowledge which was within the level of ordinary skill at the
time the claimed invention was made and does not include knowledge gleaned
only from applicant's disclosure, such a reconstruction is proper. The Cook
patent does indicate that the car shown therein is suitable for carrying
palletized loads with lift trucks being used for the loading and unloading,
including stacking of the pallets. Since the secondary references show that it
was well known to use side filler panels and bulkheads to confine palletized
loads to prevent lateral and longitudinal shifting, we agree that those
references would have suggested use of such panels and bulkheads with the
Cook car for the same purpose.
From above, it is clear that judgment on obviousness is in a sense necessarily
a reconstruction based upon hindsight reasoning, but so long as it takes into
account only knowledge which was within the level of ordinary skill at the
time the claimed invention was made, such a reconstruction is permissible.
In the present case, D5 provides a general method for the identification of a
thermodynamically stable polymorph and D6 teaches about preferred solvents
useful in the polymorphism development. Therefore, in the present case
common knowledge was readily available and combination of disclosures
taken as a whole suggest to one of ordinary skill in the art to arrive at a
thermodynamically stable form of a compound. Accordingly, there is no
question of improper hindsight in the present case.
SUBMISSIONS U/S 2(1) (ja):
It was submitted that there is neither any technical advancement nor economic
significance in the impugned invention. There is no data in the impugned
application that establishes the advantageous effects of the claimed matter
over the prior art. It was submitted that Applicant failed to provide any
comparative data with recent previous prior art compound
Applicants argument & hearing submission to opponent II
CLAIMS 2, 3, 4, 8 AND 9- OBVIOUSNESS AND LACK OF INVENTIVE
STEP [Section 25(1)(e)]
It is submitted that the question regarding inventive step is not a question of
whether the process of conversion of one polymorph into another polymorph
is obvious. The question is what are the polymorphic forms, and which form is
surprisingly superior over the other polymorphs. The discussion whether the
conversion of one polymorph into another would be obvious is not eligible
here because no polymorphic form of sorafenib tosylate is known in the prior
In general, there are substances which only appear in a single crystal form but
there are also substances which can form two, three, or even more
polymorphous crystal modifications. It is just as difficult to calculate or
predict this possible morphological and structural variety and the respective
physico-chemical, especially thermodynamic stability thereof on a scientific
mathematical basis, as it is to calculate or predict their different behaviour.
The relevant polymorphism of an organo-chemical substance is always
unpredictable in respect of the number of polymorphs, the stability thereof and
their behaviour. Due to the unpredictability, screening for polymorphs is not
routine work. The finding of new polymorphs having improved properties
does not constitute a mere discovery but fulfils all criteria needed for an
The problem is solved by the polymorphic form I which surprisingly is the
stable form among the two other polymorphs which are meta-stable. The
superior properties of polymorphic form I over polymorphic form II is
demonstrated in the mechanical stress test as per the affidavit of Dr. Britta
Olenik, a copy of which is annexed with the statement as Annexure B. In so
far as submissions made by the Opponent re Dl is concerned, it is submitted
that D1 does not give any hint as to the preparation of any sorafenib salt, let
alone of sorafenib tosylate. Accordingly, the skilled worker has no idea of
whether sorafenib tosylate is amorphous or crystalline and, in the latter case,
crystallizes in more than one crystalline modification (polymorph).
ln so far as submissions made by the Opponent re D5 is concerned, it is denied
that claim 1 is not inventive taking D1 and D5 together. It is submitted that D5
focuses on the polymorphs of compounds SK&F 94120 which is structurally
completely different from sorafenib (D5, page 529, left-hand column,
"Figure"). D5 clarifies that: "The process of crystallization and the factors
governing the appearance of individual polymorphs are still poorly
understood. Crystals are in equilibrium only with a saturated solution but
crystallisation requires supersaturation, so that the Phase Rule is not
applicable. Which of several possible polymorphs is obtained seems to depend
upon various factors: the temperature at which crystallization occurs, the
nature of the solvent (Hydrophilic, hydrophobic) and the degree of
supersaturation when crystallization commenced. The use of seed crystals can
be helpful in obtaining a desired polymorph. Manufacturing processes seem to
be worked out by trial and error aided b11 serendipity, and then adhered to
rigidly." (05, page 527, right-hand column, 2nd paragraph)
The authors further explain that:
"the methods used in attempting to obtain polymorphs include crystallization
from a range of solvents (polar and non-polar, hydrophilic and hydrophobic)
at different temperatures, for chilling solutions rapidly, by adding a second
solvent in which tire solute is sparingly soluble, by vigorously stirring excess
solid with solvent, by heati11g excess solid ∙with a high boiling solvent, by
sublimation, and by very rapidly changing the pH of a solution to precipitate
acidic or basic substances." (D5, page 528, left-hand column, 1st paragraph)
It is submitted that D5 clearly evidences that preparation and identification of
the most stable polymorph depends on a plurality of different parameters,
which might result in the desired crystal modification- with serendipity. It is
submitted that D5 clearly contradicts the submissions made by the Opponent
in paragraph under reply that the identification of polymorphs is mere routine
experimentation.
In so far as the submissions made by the Opponent re D6 is concerned, it is
submitted that it is a review article describing a conceptual approach to the
characterization of pharmaceutical solids. The document suggests four flow
charts dealing with the most common solid forms, namely polymorphs,
hydrates, desolvated solvates and amorphous forms. On eight pages, the
authors explain, how pharmaceutical solids can be characterized in a more
direct approach (D6, Abstract). The fact that in 1995, a review article has been
published in a scientific paper shows that the identification of pharmaceutical
solids in no way constituted routine experimentation. It is submitted that D6
admits that the decision trees do not necessarily lead to a result (06, page 945,
right-hand column, second paragraph). 06 is rather regarded as a guideline as
to how different forms of solids may be characterized and, if necessary,
synthesized. No reference to sorafenib tosylate can be found in D6.
Even the combination of Dl and D6 would not lead the skilled person to the
subject-matter of claim 1, since the skilled person receives no information or
guidance from D1 as to how to make different forms of sorafenib tosylate, nor
any information as to which are the polymorphs of sorafenib tosylate. The
skilled person would first have to develop the right method of preparation
before running through the four decision trees of D6. Even then, it is highly
doubtful whether the skilled person would identify polymorphic form I.
In view of what has been stated hereinabove, the Applicant submits that none
of the documents cited by the opponent (D1, D5 and D6) either alone or in
combination with the other suggest or teach the present invention nor do they
enable a person skilled in the art to arrive at the present invention.
In this regard, the Applicant also refers to and relies upon the following
scientific literature which is annexed hereto and marked as Annexure-13.
Accordingly, the subject matter of original claims 1, 2, 3, 4, 13 and 14
involve inventive step and is not obvious to a person skilled in the art as
alleged or at all (Reference in this regard is made to the pages 23 to 27
paragraphs 47 and 57 of the statement in support of the application).
The Applicant would like to draw the attention of the Learned Controller to
the following observation made by the Intellectual Property Appellate Board
in case titled Novartis AG l' Union of India & Ors Order No. 100/2009,
wherein Hon'ble Dr. P.C. Chakraborty, Technical Member, explained the
phenomenon of polymorphism as follows, which is annexed hereto and
marked as Annexure-14:
"The phenomenon of polymorphism is not universal. Its existence has to be
discovered by finding out its different forms by way of research and human
In the same order, Dr. Chakraborty further held: "It is the fact that no one
could predict the possibility of existence of polymorphism in imatinib mesylate
before the impugned application. There was no motivation also by an
uninventive man to try for finding out different polymorphic forms and their
relative properties suitable for preparing for solid dosage formulation for
cancer drug."
Applying the same principle to the facts of the present case, the applicant
submits that the possibility of existence of polymorphism in sorafenib tosylate
could not have been predicted before the present invention. In fact, none of the
cited documents either individually or collectively disclose or give any idea of
any specific polymorph of sorafenib tosylate, let alone polymorphic form I,
any pharmaceutical composition containing the same or any process for
making the said polymorphic form I.
It is further submitted that the Opponent has made assertions based on
conjecture and surmises without any cogent evidence in support thereof. In
this regard, the Applicant refers to and relies upon the case titled Raj Prakash
v 'Mangat Ram Chowdhry & Ors (R.F.A. (OS) 2 of1973) which is already
annexed hereto and marked as Annexure 9, the case titled F. Hoffman -La
Roche Ltd. V Cipla Ltd. CS (OS) 89/2008 and C. C. 52/2008 which is annexed
and marked as Annexure-9. Therefore, all the assertions and conclusions made
by the Opponent for lack of inventive step in the present representation are
wrong and are liable to be rejected in toto. The claimed subject matter
involves an inventive step and I not obvious to person skilled in the art.
After going through the submissions of both the parties it is clear
Document D1 (WO 03/068228) as cited by the opponent 2, also disclosed in
the impugned patent specification discloses tosylate salt of sorafenib and its
use in the treatment of disorders in which angiogenesis plays an important role
for example in tumor growth. The teaching that flows from this document is
that tosylate salt of sorafenib is known.
D5 as cited by the opponent is research article published in Chemistry &
industry, 1989, pages 527-529 is a general article which discloses the
common general knowledge in the field of polymorphism surely discloses
that "The thermodynamically stable polymorph needs to be identified. If the
compound is enantiotropic, there will be two or more stable polymorphs and
transition temperatures as well. These can be identified by simple
techniques, for example by stirring or shaking excess solid with solid at
different temperatures. "(See on page 528, column 02). The same article also
on page 527, column 02 under section "Crystals and Crystallisation", "The
use of seed crystals can be helpful in obtaining a desired polymorph.
Manufacturing processes seem to be worked out by trial and error aided by
serendipity, and then adhered to rigidity. "
The teaching that certainly flows from this article clearly suggests that stirring
or shaking and seeding technology is very well known in the art prior can be
used to prepare a thermodynamically stable polymorph.
Thus a person skilled in the art can easily combine the teachings D1& D5 to
arrive at thermodynamically stable polymorph 1 of sorafenib tosylate.
Therefore claim 1 lacks inventiveness.
Claims 2, 3, 4, 8 and 9 relates to the conversion of one polymorph to another
thermodynamically stable polymorph, particularly a process for the
conversion of polymorph-II to polymorph-I.
The disclosure on page 15 & 16 on the specification of the impugned
invention discloses the process for the conversion of polymorph-II to
polymorph-I of Sorafenib tosylate; "Preference is given to preparing the
compound of the formula (I) in the polymorph I by effecting the compound of
the formula (I) in the polymorph (1l) obtained as described in example 1, in
methanol, ethanol, a mixture of both solvents or a mixture of both solvents
with water, preferably a 1:1 mixture with water, and shaking or stirring at a
temperature of from 50°C up to the reflux temperature of the
Solvent, preferably at from 60 to 80°C, in the absence of crystals of a solvate
of the compound of the formula (I), for example in the absence of crystals of
the methanol solvate or the ethanol solvate of the compound of formula (I),for
up to one day. The crystals are cooled to from -30°C to room temperature,
preferably from -25 to 1 0°C, isolated and dried The compound of the formula
(I) is thus obtained in the polymorph I Most preferably isopropanol, ethyl
acetate or a mixture thereof is used as solvent.
"Preference is likewise given to preparing the compound of the formula (I) in
the polymorph I by effecting the compound of the formula (I) in the polymorph
II, obtained as described in example 1, in methanol, ethanol, a mixture of both
solvents or a mixture of both solvents with water, and shaking or stirring at a
temperature of from 1 0°C up to the reflux temperature of the solvent,
preferably at room temperature, for up to 1 day. The mixture is subsequently
seeded with crystals of the compound of the formula (I) in the polymorph I and
stirred or shaken, for example at room temperature, for from 1 hour to 14
days, preferably from 2 hours to 7 days. The crystals are isolated and dried.
The compound of the formula (1) is thus obtained in the polymorph I most
preferably isopropanol, ethyl acetate or a mixture thereof is used as solvent"
Thus from the disclosures Dl in view of D5 it is clear that, polymorph-I of
Sorafenib tosylate is obtained by heating the polymorph-II of Sorafenib
tosylate in an inert solvent. Subsequently, it mentions that seeding is preferred
for obtaining the polymorph-I of Sorafenib tosylate.
Also referring to the disclosure page 02, first paragraph of the impugned
application & the admittance of the fact that Sorafenib tosylate of Dl exist in
polymorph-II, starting from Dl conversion of a known compound to another
thermodynamically stable polymorph certainly obvious in view of
D5.Therefore the process as claimed in the claims 2, 3, 4, 8 and 9 of impugned
application involves no inventive step of any sort and does not satisfy the
criteria of non-obviousness.
Document D6 relates to review article published Pharmaceutical Research,
vol. 12, No. 7, pages 1995, 945-954 provides a review of strategic approaches
to remove much of uncertainty by presenting concepts and ideas in the form of
flow charts to control the crystal form (polymorph) of drug substance. D6
provides a review of strategic approaches to remove much of uncertainty by
presenting concepts and ideas in the form of flow charts to control the crystal
form (polymorph) of drug substance.
It outlines investigations of the formation of polymorphs and the controls
needed to ensure the integrity of the drug substance containing either a single
or mixture of polymorphs. D6 on page 946, under the heading; "Formation of
Polymorphs - Have Polymorphs Been Discovered?"The first step in the
polymorph decision tree is to crystallize the substance from a number of
different solvents in order to answer the question: Are polymorphs possible?
Solvents should include those used in the final crystallization steps and those
used during formulation and processing and may also include water,
methanol, ethanol, propanal, isopropanol, acetone, acetonitrile, ethyl acetate,
hexane and mixtures if appropriate. New crystal forms can often be obtained
by cooling hot saturated solutions or partly evaporating clear saturated
solutions. . "
The teaching that flow from this document D6 is the use of specific solvents
during preparation of polymorphs & specifically mentions the use of solvents
such as water, methanol, ethanol, propanal, isopropanol, acetone, acetonitrile,
ethyl acetate, hexane and mixtures. Here I see that same solvents has been
used by the applicants in preparing the polymorph I of Sorafenib tosylate in
the working examples of the impugned application
Thus by combining teachings of D5 & D6, a skilled artisan it is practically
possible to arrive at a process of converting one polymorph to another
polymorph using the solvents mentioned in the D6. Thus process claims lack
inventive merit.
GROUND 8: CLAIMS NOT PATENTABLE UNDER SECTION 3(d)
Opponent I & hearing submission
(a) The amended claims are drawn to polymorphic form 1 of Sorafenib
tosylate (4-(4-[({[4-chloro-3-trifluoromethy) phenyl] amino} carbony) amino]
phenoxy)-N-methylpyridine-2-carboxamide (hereinafter referred to as
"polymorph of Sorafenib tosylate"). It is submitted that the claims are not
patentable under Section 3(d). In the case of Novartis vs. Union of India [AIR
2013 SC 1311], the Supreme Court was concerned with the patentability of
polymorphic form of imatinib mesylate. It was contented by the
Applicant/Petitioner, Novartis before the Supreme Court that the crystal form
has better physical properties including that it is thermodynamically more
stable, less hydroscopic and has lower hygroscopicity (page 63, para 168).
This was supported by expert evidence submitted by the Appellant-Novartis.
Comparison was made of these physical properties of the crystal form with the
base and it was contended that because the crystal form exhibits better
solubility, the same should be taken as a measure of increased bioavailability
and hence, improved therapeutic efficacy. It was contended that the crystal
showed 30% improvement in bioavailability as compared to the free base
(para 168-169, page 63). The Supreme Court considered these submissions in
paragraphs 175-190 and at paragraph 180, especially paragraph 187 the
Supreme Court has held that "In whatever way therapeutic efficacy may be
interpreted, this much absolutely clear that the physic-chemical properties of
beta crystalline form of Imatinib Mesylate, namely (i) more beneficial flow
properties, (ii) better thermodynamic stability, and (iii) lower hygroscopicity,
may be otherwise beneficial but these properties cannot even be taken into
account for the purpose of the test of Section 3(d) of the Act, since these
properties have nothing to do with therapeutic efficacy". The Supreme Court
has also held in paragraph 188 that "Bioavailability falls outside the area of
efficacy in case of a medicine….". Further, in paragraph 189, the Court held
that "… the position that emerges is that just increased bioavailability alone
may not necessarily lead to an enhancement of therapeutic efficacy….". The
Supreme Court has also held that "… whether or not an increased in
bioavailability leads to enhancement of therapeutic efficacy in any given case
must be specifically claimed and established by research data….".
In other words, the Supreme Court has rejected mere comparison of
base with a claimed form as sufficient for establishing therapeutic efficacy and
held that therapeutic efficacy must be established by submitting appropriate
research and clinical data.
In the case at hand, the claimed polymorph 1 fails to pass the test of
Section 3(d) as interpreted by the Supreme Court because:
No material on record to establish therapeutic efficacy: Apart from the
averments in the Reply statement, there is no document or material on record
to establish therapeutic efficacy as required by the Supreme Court. No
research data is presented to fortify the conclusion that polymorph 1 exhibits
better therapeutic efficacy. There is nothing in the patent application to
support this conclusion.
Affidavits of Britta Olenik and of Kerstin Pauli do not provide any
research data to support therapeutic efficacy: These affidavits submitted by the
Applicant do not further the case of the Applicant insofar as therapeutic
efficacy is concerned. They only compare the dissolution rate of Sorafenib
tosylate salt with the base Bay-43-9006. Dr. Pauli on the basis of better
dissolution rate of the polymorph 1 concludes that the efficacy of the said
polymorph 1 is higher than Sorafenib base. The conclusion is not reached on
the basis of any research data as required by the Supreme Court, and is a mere
conclusion without any basis on data.
(iii) Affidavits defective, unreliable:
Even otherwise, the dissolution rate as presented by these experts cannot
automatically lead to an inference of better therapeutic efficacy because the
affidavits do not present any data for the same.
Affidavit of Dr. Kerstin Pauli: this compares the formulation of Sorafenib
(BAY 43-9006 micron) and formulation of Sorafenib tosylate polymorph I
(BAY 54-9085 micron). Prima facie, it may be noted that both formulations
are not similar and that the quantity of Avicel PH101 is much higher when the
formulation is based on Sorafenib. Perhaps, the quantity of Avicel has been
increased in the Sorafenib based formulation in order to match the weight of
the tablet containing Sorafenib tosylate polymorph I. Avicel by itself has an
effect on the dissolution property of the tablet and an increased quantity of a
Avicel decreases the rate of disintegration and thereby the dissolution. Such
difference has not been accounted for in the final result i.e. there is nothing to
suggest that the high dissolution is only on account of Polymorph-1 and not
Non-disclosure of conditions nor the experiment: Further, the conditions under
which the dissolution test was conducted are not disclosed in the affidavit.
No data to show that improved dissolution = enhanced efficacy: The results of
the dissolution studies are only limited to the dissolution of the samples and
there is no data to show how the said improvement in the dissolution would
actually result in increased safety or efficacy. No automatic assumption of
therapeutic efficacy can be made unless substantiated by "research data"
Comparison of base with polymorph is inappropriate – real comparison is
between two polymorphs: Dissolution of the Sorafenib tosylate polymorph I
over that of the base cannot demonstrate any efficacy- the real the standard for
comparison has to be Sorafenib tosylate polymorph II being the closest prior
Affidavit of Dr. Britta Olenik: In this affidavit, it is stated that the polymorph I
of Sorafenib tosylate was subject to mechanical stress, i.e. by grinding in hand
for about 30 seconds and compared with unground or untreated samples.
Similarly, polymorph II was also compared before grinding and after grinding.
This affidavit does not depict when the data was measured and does not rule
out automatic conversion from form II to form I. This comparison is nothing
but comparison of a physicochemical parameter i.e. stability of the
polymorphic form. There is nothing data to conclude that such stability
contributes to therapeutic efficacy which is the requirement for patentability
under Section 3(d).
Assuming but not conceding that polymorph II is more sensitive to
mechanical stress in comparison of polymorph I, a measure of mechanical
stress cannot be said to contribute or translate into therapeutic efficacy, which
is required under Section 3(d).
(d) Comparison of base versus polymorph-1: Not proper and does not
satisfy Section 3(d) requirements
As mentioned above, the Supreme Court has already laid down that
therapeutic efficacy must be established by submission of appropriate data. In
the case at hand as well as in the case before the Supreme Court, the
dissolution rate was presented as a measure of enhanced bioavailability and
comparison was made of the polymorph with the base. Such comparison was
specifically rejected by the Supreme Court in paragraph 188. Therefore, the
comparison prima facie is against the law laid down by the Supreme Court.
Bioavailability does not automatically mean therapeutic efficacy:
It was contended that bioavailability is a measure of therapeutic efficacy. In
this regard, it is submitted that therapeutic efficacy and bioavailability are two
different parameters and are required to be specifically demonstrated through
data. Bioavailability is pharmacokinetic parameter whereas therapeutic
efficacy is a pharmacodynamic parameter. Even as per the Supreme Court,
therapeutic efficacy must be specifically establish through data, which has not
been put forth in the present case.
The impugned application makes no claim of enhanced therapeutic
The specification of the impugned application sets out the properties and
advantages of polymorph 1. It clearly states that the invention is drawn to a
thermodynamically stable form of Sorafenib tosylate salt. The inventive
feature is the finding of the polymorphic form 1, which is stable enough and
does not convert into another polymorphic form and prevents changes in
solubility or bioavailability profile. Nowhere is it contended in the entire
specification that the polymorph 1 of Sorafenib tosylate salt exhibits better
therapeutic efficacy and treats/contributes to an improvement in the treatment
of cancer. Obviously, the Applicant at this stage of the Opposition cannot
advance contentions contrary to or not pleaded in their own specification.
In view of the above, it is submitted that the claims drawn to polymorph 1 of
Sorafenib tosylate salt are not patentable and liable to be rejected.
Applicants Argument to Opponent I
With regard to Section 3(d) of the Patents Act, it is submitted that the
assertions made by the Opponent in the present representation are based on
conjectures and surmises without corroborative evidence in support thereof.
It is submitted that it is a settled proposition of law that it is for the Opponent
to discharge the onus that inter alia, the invention claimed by the Applicant is
new form of the known substance and mere submissions will not
axiomatically permit the Court to believe that the invention claimed by the
Applicant is new form of the known substance unless shown clinically with
some evidence. It is submitted that in the present case, the Opponent has failed
to corroborate its submissions by placing some cogent evidence on record and
therefore, has terribly failed to discharge its onus. On this ground alone, the
present opposition deserves to be rejected outrightly. In support of its
submissions, the Applicant refers to and relies upon the case titled F.Hoffman
-La Roche Ltd. V Cipla Ltd. CS(OS) 89/2008 and C.C. 52(2.008 which is
annexed hereto and marked as Annexure-6.
It is further submitted that the assertions made by the Opponent in the present
representation are misleading, lack proper interpretation of law, science and
are based on assumptions and presumptions and therefore, are vehemently
denied. The Opponent is once again wrong when he states that the present
claims 1 to 18 are drawn to polymorphic forms of 4-{4-[({[(4-chloro-3-
trilluoromethyl) phenyI] amino} carbonyl) amino] phenoxy} -N-
methylpyridine-2-carboxamide which is sorafenib. The correct statement
would be that the present claims relate to the polymorphic forms of the
tosylate salt of sorafenib which is different from sorafenib itself.
The efficacy of the tosylate salt of sorafenib in polymorphic form is higher
than its base sorafenib due to a higher dissolution in tablets containing both
drugs each. In, this regard, the Applicant refers and relies upon the evidence in
the form of technical affidavit deposed by Dr. Kerstin Pauli that was
submitted along with the statement in support of the application. Therefore,
the present invention is patentable and none of the claims fa1ls within the
prohibition of Section 3(d).
Furthermore, it is very important for a pharmaceutical product to always have
the same constant properties to guarantee constant and reliable efficacy. There
is a need to find the most stable form of a compound because only the most
stable form can ensure that all properties and characteristics regarding
stability, dissolution rate, shelf life, efficacy, and bioavailability remain
constant during manufacturing, storage, and administration. The polymorphic
form I of sorafenib tosylate of the present invention is surprisingly more stable
than the other polymorphs found and ensures a constant & reliable efficacy,
therapeutic or otherwise. 1n this regard, the Applicant refers to & relies upon
the evidence in the form of technical affidavit deposed by Dr. Britta Olenik
that was submitted along with the statement in support of the application.
Therefore, the present invention is patentable and none of the claims fall
within the prohibition of Section 3(d). When the polymorphic form I of
tosylate salt of sorafenib itself is patentable, its preparation ought to be
patentable as well.
Opponent II Arguments & hearing submission
It was submitted that the subject matter of the impugned application is not an
invention within the meaning of Section 3(d) of the Act because the claimed
invention falls within the mischief of Section 3(d). It states that:
"the mere discovery of a new form of a known substance which does not result
in the enhancement of the known efficacy of that substance or the mere
discovery of any new property or new use for a known substance or of the
mere use of a known process, machine or apparatus unless such known
process results in a new product or employs at least one new reactant.
Applicant relies upon affidavit of Dr. Kerstin Pauli to fulfill the requirement
of Section 3(d). Opponent submitted that reliance to the affidavits of Dr. Pauli
and Dr. Olenic should not be taken due to the reasons mentions under
preliminary submissions.
Opponent submitted that, in any case if reliance can be taken to these
affidavits, still the requirements U/S 3(d) are not fulfilled. Affidavit of Dr.
Pauli shows the comparative dissolution data of Sorafenib free base and
Sorafenib tosylate.
In this regard, Opponent referred to Hon'ble Supreme Court
decision on Gleevac case, which was handed out during the hearing. The
relevant paragraphs there from are set-out for ready reference:
Page 88, Para 171:
"That being the position, the appellant was obliged to show the enhanced
efficacy of the beta crystalline form of Imatinib Mesylate over Imatinib
Mesylate (non-crystalline). There is, however, no material in the subject
application or in the supporting affidavits to make any comparison of efficacy,
or even solubility, between the beta crystalline form of Imatinib Mesylate and
Imatinib Mesylate (non-crystalline).
Drawing analogy for the instant case, there is no comparison between
Sorafenib tosylate prepared by general standard method i.e. D1 with
Sorafenib tosylate of present claims.
Affidavit of Dr. Pauli shows that there is difference in dissolution profile of
Sorafenib and sorafenib tosylate Form-I.
In this regard, it was submitted that, salt formation is the most common and
effective method of increasing solubility and dissolution rates of acidic and
Also, Supreme Court made it clear that
172. As regards the averments made in the two affidavits, for all one knows
the higher solubility that is attributed to the beta crystalline form of Imatinib
Mesylate may actually be a property of Imatinib Mesylate itself. One does not
have to be an expert in chemistry to know that salts normally have much better
solubility than compounds in free base form. If that be so, the additional
properties that may be attributed to the beta crystalline form of Imatinib
Mesylate would be limited to the following:
i. More beneficial flow properties,
ii. Better thermodynamic stability, and
iii. Lower hygroscopicity
173. The aforesaid properties, ("physical attributes" according to Manley),
would give the subject product improved processability and better and longer
storability but, as we shall see presently, on the basis of those properties
alone, the beta crystalline form of Imatinib Mesylate certainly cannot be said
to possess enhanced efficacy over Imatinib Mesylate, the known substance
immediately preceding it, within the meaning of section 3(d) of the Act (see
Page 88).
Therefore, requirements of section 3(d) are not fulfilled. Hence, ought to
be rejected on this ground too.
Additionally, affidavit of Dr. Olenik has been cited by the applicant.
However, it is submitted that Mechanical stress is not a criterion to show
efficacy of a drug.
Supreme Court on Page 94, 187 further states that:
187. In whatever way therapeutic efficacy may be interpreted, this much is
absolutely clear: that the physico-chemical properties of beta crystalline form
of Imatinib Mesylate, namely (i) more beneficial flow properties, (ii) better
thermodynamic stability, and (iii) lower hygroscopicity, may be otherwise
beneficial but these properties cannot even be taken into account for the
purpose of the test of section 3(d) of the Act, since these properties have
nothing to do with therapeutic efficacy.
Last lines of para 188:
"It is not the intent of a bio-availability study to demonstrate effectiveness, but
to determine the rate and extent of absorption. If a drug product is not
bioavailable, it cannot be regarded as effective. However a determination that
a drug product is bio-available is not in itself a determination of
effectiveness."
Opponent submitted that, since Supreme Court has provided clear directions
to overcome the barrier of Section 3(d). However, in the present case
Applicant failed to submit such data and therefore deserves to be rejected
Applicants argument & hearing submission to Opponent II
With regard to Section 3(d) of the Patents Act, it is submitted that
the assertions made by the Opponent in the present representation are
based on conjectures and surmises without corroborative evidence in
support thereof. It is submitted that it is a settled proposition of law
that it is for the Opponent to discharge the onus that inter alia, the
invention claimed by the Applicant is new form of the known
substance and mere submissions will not axiomatically permit the
Court to believe that the invention claimed by the Applicant is new
form of the known substance unless shown clinically with some
evidence. It is submitted that in the present case, the Opponent has
failed to corroborate its submissions by placing some cogent
evidence on record and therefore, has terribly failed to discharge its
onus. On this ground alone, the present opposition deserves to be rejected
outrightly. In support of its submissions, the Applicant refers to and
relies upon the case titled F. Hoffman -La Roche Ltd. V Cipla Ltd.
CS(OS) 89/2008 and C.C. 52/2008 which is already annexed hereto and
marked as Annexure-15.
It is further submitted that the assertions made by the Opponent
in the present representation are misleading, lack proper
interpretation of law, science and are based on assumptions and
presumptions and therefore, are vehemently denied.
It is submitted that the efficacy of the tosylate salt of sorafenib in
polymorphic form l is higher than its base sorafenib due t o a higher
d i ss o lu tio n in tablets containing both drugs each. I n this regard, the
Applicant refers and r e l i e s upon the evidence in the form of technical
affidavit deposed by deposed by Dr. Kerstin Pauli, a copy of which is
annexed with the statement as Annexure c
Furthermore, it is very i m p o r t a n t for a pharmaceutical product to
always have the same constant properties to guarantee constant and
reliable efficacy. There is a need to find the most stable form of a
compound because only the most stable form can ensure that all
properties and characteristics regarding stability, dissolution r a t e, shelf
life, efficacy, a n d bioavailability rem ain constant
manufacturing, storage, and administration. The polymorphic form I of
sorafenib tosylate of the present invention is surprisingly more
stable than the other polymorphs found and ensures a constant and
reliable efficacy, therapeutic or otherwise. In this regard the
Applicant refers to and relies upon the evidence in the form of
technical affidavit deposed by Dr. Britta Olenik, which is annexed with
the statement as Annexure B.
60. Therefore, the present invention is patentable and none of the
claims fall within the prohibition of Section 3(d). When the
polymorphic form I of tosylate salt of sorafenib itself is patentable,
its preparation ought to be patentable as well.
GROUND 9: U/s 25(1) (h), THE FAILURE TO DISCLOSE DETAILS
OF CORRESPONDING FOREIGN APPLICATIONS;
Opponent I arguments & hearing submission
The patentee has failed to disclose to the Controller the information required
under Section 8: It is submitted that the Applicant-Respondent has failed to
disclose the details of corresponding foreign applications filed and on this
ground alone the patent application should be rejected.
The applicant is required to provide all the information regarding the
prosecution of the equivalent applications till the grant of the Indian
application to the Controller in writing from time to time and also within the
prescribed time. The applicant has failed to furnish the details of National
phase applications filed in USA, Europe etc, which are still under examination
and not granted. Therefore the applicant has failed to comply with the
requirements of the section 8 of the act and the opponent demands rejection on
this ground also.
Opponent II arguments & hearing submission
Opponent II has not pressed this ground.
Applicants arguments & hearing submission to Opponent I
The averments made by the Opponent are wrong and denied in toto. It is
submitted that the requirement under Section 8(1)was fulfilled by providing a
list of corresponding foreign applications on Form-3 dated, March 14,
2007,August 9, 2012, February 15, 2013, August 12, 2013, March 04, 2014,
and August 12, 2014. It is further submitted that the requirements under
Section 8(2) were only requested by the learned Controller at the time of
issuance of the First Examination Report (FER) dated September 16, 2013,
which as per the provisions of the Patents Act was required to be complied
with within six months from the date of request, i.e., March 17, 2014. The
Section 8(2) details were submitted by the aforesaid deadline under cover of
our letter dated March 04, 2014.
Without prejudice to the above, it is submitted that requirements under Section
8 need to be complied with until the grant of a patent and considering that the
patent application is still pending, the said ground taken by the Opponent is
pre-mature and ought not to be taken cognizance of. Therefore, the Applicant
has complied with the information required under Section 8 of the Act, and the
ground taken by the Opponent is wrong and frivolous (Reference in this
regard is -made to the page 12, paragraph 43 of the statement in support
of the application).
It is submitted that in this regard, the Applicant refers to and relies upon the
case titled Sukesh Behl v. Koninklijke Philips Electronics [FAO (OS) No.16
OF 2014] which is annexed hereto and marked as Annexure-11.
It is submitted to the Learned Controller that the requirements under Section 8
are mere procedural requirements and based on doctrine of duty of candour.
As long as the Applicant is not trying to mislead the Learned Controller, the
Learned Controller ought not to take into consideration the grounds of
opposition based on Section 8.
In any case, since the Section 8(2) details were submitted within the
prescribed time period, it is submitted that the ground taken by the Opponent
is wrong and frivolous.
OTHER MISCELLANEOUS GROUNDS
REBUTTAL TO THE ARGUMENTS OF THE APPLICANT BY THE
OPPONENT II:
(i) Rejection of Pre-grant Opposition as no evidence was filed.
The Applicant has argued that the pre-grant opposition has not
been accompanied by any expert evidence and therefore, none of the
arguments made by the Counsel should be considered. In this regard, it is
(a) Filing of evidence is not mandatory:
As per Section 25(1) of the Patents Act, read with Rule 55(1), any person
may file a representation accompanied by evidence, if any. The words "if
any" in Rule 55 are very important and denote that the evidence may
be filed, if required. Filing of evidence is not mandatory. The law must be
read only in this manner because the Patent Office and the Controller are
specialized tribunals, empowered to adjudicate upon technical issues by
themselves as they are experts in certain technical fields unlike a court of
law, wherein the judges are by and large from a legal background.
In addition, if the grounds taken in Opposition are such as anticipation, non-
compliance of Section 8 etc., the arguments advanced under these grounds
are purely legal and factual. There is no requirement for any expert to
intervene in these grounds and provide his expert opinion. Such expert
evidence may at best be required to substantiate a ground of obviousness.
Even for this ground if the arguments are fairly simplistic and the
controller is fairly capable of adjudicating thereon without external aid,
there is no need for any expert evidence. It must be borne in mind that the
pre-grant opposition is a summary proceeding unlike a post-grant opposition.
In the case of Ajanta Pharma Limited Vs. Allergan inc. & Ors. The IPAB
has held in paragraph 15 that if the novelty and inventive merit of an
application can be destroyed by well published literature then, the evidence is
(b) Evidence may be by way of documents or affidavit:
As per the Evidence Act, Section 3, evidence may be of two types –
documents and affidavit, i.e. documentary evidence and evidence by way of
affidavit. Documentary evidence is given when a person wants to rely on the
contents of that document.
The document is relied upon for the content of that document and for such
contents, only the author of the document can depose as to the veracity of
that document and not any third party to the document including the
opponent. Therefore, it is futile to demand an affidavit from this Opponent
in respect of the contents of a paper authored by a third person.
In any case, Evidence Act allows any person to rely on evidence which may
be mere documents. In all court proceedings, or before tribunals, parties
often rely on documents which may be extracts from dictionary, scientific
papers, internet extracts and all of these are admissible under the category
‘documentary evidence'.
Even otherwise, as per the Patents Act Section 129 read with rule 126,
affidavit can only be given in respect of facts which are personal and which
are known to the deponent. In the present case, the facts which emanate
from the documents are set out in the pre-grant representation and this
requirement of law is satisfied. The Opponent is relying on documentary
evidence which satisfy the requirement of Rule 55(1).
(c) JUDGEMENTS RELIED UPON BY APPLICANT
The Applicant relied upon the judgments Molnlycke AB Vs. P&G
(1994 R.P.C. 49), Stix Limited Vs. Maharaja Appliances Limited, Roche Vs.
Cipla, Reynolds Vs. Herbet Smith and Co. in support of the proposition but
evidence by way of affidavit must be provided.
None of these are applicable in the present case as none of these
judgments relate to pre-grant proceedings. They are judgments rendered in
the context of infringement proceedings in a court of law which does not
include technical expert as judges.
In view of the above, the preliminary objection raised by the Applicant is
not tenable in law and deserves to be rejected.
(ii) PROOF OF LACK OF INVENTIVE STEP IN PRE-GRANT
PROCEEDING VERY HIGH, NOT MET BY OPPONENT.
The argument of the Applicant that the threshold of inventive step is pre-grant
opposition is far higher than revocation of post-grant proceedings, is
without any merit. The use of the words "clearly does not involve
inventive step" denotes clarity. The Opponent is required to prove his case
clearly, without a doubt. That, certain does not mean that the proof of lack of
inventive step is higher. No grades or degree of proof is prescribed in law
and cannot be read into it. This argument of the Applicant must therefore, be
Applicant's contention to Opponent I
PRELIMINARY SUBMISSIONS
(A) Reliance placed by the Opponent on Novartis AG v Union of India &
Ors. (MANU/SC/0281/2013)- Misplaced:
At the very outset, it is submitted that the reliance placed by the Opponent on
case law titled Novartis AG v Union of India & Ors. Civil Appeal nos.
2706-2716 of 2013 which is annexed hereto and marked as Annexure-I is
misplaced. In this regard, the attention of the Learned Controller is drawn to
paragraph 191 of the said judgment which is reproduced herein below
for the Learned Controller's ease of reference:
We have held that the subject product, the beta crystalline form of imatinib
Mesylate, does not qualify; the test of Section 3(d) of the Act but that is not
to say that Section 3(d) bars patent protection for all incremental inventions
of chemical and pharmaceutical substances. It will be a grave mistake to read
this judgment to mean that section, 3(d) was amended with the intent to undo
the fundamental change brought in the patent regime by deletion of section
5 from the Parent Act. That is not said in this judgment.
It is submitted that from the observation made by the Hon'ble Supreme Court
of India, it is evident that the Hon'ble Court made it clear that the
observations made therein were specific to the subject matter before it and as
such, the said case law cannot be interpreted to mean that it bars patent
protection for all incremental inventions of chemical and pharmaceutical
substances. Each case has to be decided on its own merits and
therefore1 the observations made therein will not have the blanket
application as has been the understanding of the Opponent. For the reasons
detailed out herein below/ it is submitted that the said case law has no
application, whatsoever, to adjudicate upon the issues involved in the matter.
(B) Grounds of Pre Grant the present representation are Frivolous:
2. The Applicant humbly submits that the grounds on which the present
representation has been filed by the Opponent and the argument set forth
therein are frivolous in that they have not fathomed and appreciated the
Applicant's invention, as will be evident in the Applicant's corresponding
submissions to the statement which are enumerated herein below for the
Controller's just consideration.
3. At this stage, it is pertinent to draw the attention of the Learned Controller
that the Opponent in the present has failed to annex the amended and/ or
c l a i m s under opposition, it can safely be concluded that the submissions
made by the Opponent in the present representation are based on
conjectures and surmises and are baseless. On this ground alone, the
present representation ought to be rejected.
(C) No evidence filed by the Opponent to establish lack of inventive step:
o f t h e p a t e n t s Act,1970 (hereinafter referred to as "the Patents
Act") mandates that evidence before the Controller should be in the form of
an affidavit. It is further submitted that the issue as to whether the subject
patent application lacks inventive step' or not cannot be decided by making
mere submissions which in either event are based on conjectures &
surmises in a manner as been made by the Opponent in the present
5. Without prejudice to the above, it is submitted that it is a settled
proposition of law that in order to assess obviousness, the Court would
invariably require the assistance of expert evidence. In this regard the
Applicant refers to and relies upon the case titled M6lnlycke v Proctor &
Gamble. [1994] R.P.C. 49 at page 113 which is annexed hereto and marked
as Annexure-2 and Stix Limited v Maharaja Appliance Limited (l.A. No.
7441 of 2008 in CS (OS) No. 1206 of 2008 which is annexed hereto and
marked as Annexure·3.
6. In the present case, the Opponent has failed to corroborate the submissions
by placing cogent evidence in the form of an affidavit on record as mandated
by Section 79 of the Patents Act and also being the settled proposition of
law. In view of the above, the present representation ought to be outrightly
(D) Threshold of inventive step much higher in Opposition proceedings than
in case of revocation proceedings:
m u c h h i g h e r in Opposition proceedings than in revocation proceedings.
In case, the Learned Controller has an iota of doubt regarding the
inventive step, the Learned Controller should allow the grant leaving the
question to be finally decided, when an occasion arises, by Intellectual
Property Appellate Board (IPAB) and/ or the High Court. In "Patent Law" by
P. Narayanan, Fourth edition, the author has at pages 403 and 213 (which
is annexed hereto and marked as Annexure distinguished the inventive step
requirement in case of an opposition proceeding and in case of a revocation
proceeding as under:
"The words "clearly does not involve any inventive step" in Section 25(1)(e)
would appear to indicate that in an opposition proceeding the
evidence required to establish lack of inventive step would be more
stringent."
''Under Section 64(1) (j) obviousness and lack of inventive step is also
available as a ground of revocation of a patent by petition before the
High Court. In opposition proceedings under Section 25(1) (e) and Section
25(2) (e) it must be shown that the invention "clearly" does not involve any
inventive step ∙while there is no such qualification under Section 64(1)(}).
This shows that if the matter is in doubt, the Controller may allow the grant
leaving the question to be finally decided, when an occasion arises, by the
High Court."
(E) Scheme of the Patents Act- The Opponent ought to have put the best case
It is submitted that before the Learned Controller adjudicates upon the
present representation by way of an opposition filed by the Opponent against
the grant of patent of the patent application, it is pertinent to draw the
attention of the Learned Controller to the relevant provisions of the Patents
Act which will provide an insight of the scheme of the Patents Act. It is
submitted that prior to the January 1, 2005, there was no concept of post-
grant opposition and Section 25 provided opposition to grant of patent. It is
submitted that the Section 25(1) of the Patents Act read with Rule 55 of
Patents Rules, 2003 (hereinafter referred to as "the Rules") contemplates
representation by way of an opposition which is a diluted form
of representation.
Rule 55 of the Rules provides the procedure for pre-grant Opposition under
Section 25(1) of the Act. Rule 55(1) mandates the Opponent to file statement
and evidence, if any, in support of the representation. Rule 55(3) states that
on consideration of the representation, if the Contro11er is of the opinion that
the application for patent shall be refused or the complete specification
requires amendment, 'he shall give notice to the Applicant to that effect along
with a copy of such representation, whereupon, the Applicant, as per Rule
55(4), shall file his statement and evidence, if any, in support of his
application within three months from the date of notice. In view of the above,
it is evident, that what is contemplated for the Applicant under Rule 55 (4) of
the rules is to file statement and evidence in support of his application
and not reply statement (which is the case re post-grant opposition
proceeding i.e., reply to written statement filed by the Opponent as
contemplated under Rule 58 of the Rules re post-grant opposition
proceeding). Accordingly, each of the parties is required to put is best case
in support of its respective submissions.
It is further submitted that as the Rules are silent on filing of a rejoinder by
the Opponent in response to the statement and evidence of the Applicant
in support of the application and also on filing of the reply evidence by the
Opponent as contemplated under Rule 59 of the Rules re post-grant
opposition, the Opponent ought to put the best case forward, which, in the
absence of any cogent evidence in the form of an affidavit, as mandated by
Section 79 of the Act, the Opponent has miserably failed.
It is submitted that the submissions made in the patent application/ complete
Specification are made on oath in as much as Form 1 mandates a declaration
to be made by the Applicant therein. In view thereof, there can be no doubt on
the credibility of the Applicant.
Without prejudice to the above, it is submitted that the patent application in
question discloses and claims subject matter comprising type I polymorph of
sorafenib tosylate having higher thermodynamic stability, mechanical
stability, and dissolution rate than sorafenib tosylate type II polymorph. The
polymorph II crystals are meta-stable in nature. In general meta-stable
polymorphs tend to react with surrounding molecules like carrier
molecule, other constituents of pharmaceutical compositions, humidity and
the like. For example, the meta- stable state of sorafe.nib tosylate tends
to convert itself into more stable structure, which may eventually lead to
change in physical characteristic
like dissolution rate, solubility,
absorbability and the like of sorafenib tosylate. That the change in physical
characteristics of the molecule will affect the therapeutic efficacy of the
molecule is well understood in art.
The tendencies of meta-stable compounds to convert into different
structures by exhibiting polymorphism or otherwise, or to form different
compounds by reaction with other compounds, are not desirable due to
changes in the physical and chemical properties of the pharmaceutical
compounds, which in turn determines the therapeutic properties of a drug.
Therefore, compounds in their most stable form are required for efficacious
and stable drug formulation.
It is submitted that the thermodynamic polymorphs of a compound are a
solution to such meta-stability issues of a compound. However, the
determination of stable structure and preparation of polymorphs itself takes
considerable effort and intellectual investment. Furthermore, although
d e t e r m i n a t i o n a n d formation of polymorphs that suits a particular
pharmaceutical application is in itself a mammoth task and involves
substantial intellectual and financial investment.
Opponent II rebuttal to Applicants preliminary submissions
Preliminary Submissions
Amended set of claims
Form of an Affidavit
Threshold in opposition proceedings
Opponent's submissions on Admissions made by the Applicant
Opponent's submissions on Affidavits filed by the Applicant
The "Statement and Evidence in support of the application U/S 25(1) of the
Patents Act, 1970 read with Rule 55(4) of the Patent Rules, 2003" submitted
by the Applicant on January 19, 2015 is hereinafter referred as "Statement and
Evidence" and the Representation U/S25 (1) read with rule 55 of the Act is
hereinafter referred as "Representation".
Amended set of claims:
It was submitted that there is no alteration to the scope of the current set of
claims. Therefore, present representation automatically addresses the subject
matter of the pending claims and there is no need to file a fresh or
supplementary representation. The newly inserted claims 14-16 dependent on
Claim 1 cover characteristic details of compound of claim 1 and merit no
Form of an affidavit
Applicant raised several questions under the principles of C.P.C on the
admissibility of the affidavits filed by the Opponent.
It was submitted that at this juncture, we need to observe that the principles of
C.P.C and the decisions given thereon may not always be applicable in a
patent or trademark litigation, more particularly in a patent litigation. We may
in civil litigation on the particular facts of the case refuse to receive the
evidence and documents, if they are belatedly or improperly produced before
But the same principle cannot be applied here. It may noted be that the ‘any
person' in opposing the grant or interested in revoking the grant had belatedly
come across a prior art which squarely anticipates the patent or renders the
patent obvious. The Controller cannot shut out the documents merely on the
ground of delay.
Similarly, if affidavits submitted by the Opponents which squarely anticipate
the application for patent finds some legal deficiencies, it cannot be rejected at
It was submitted that a patent is granted only for an invention, which is a new
product or process involving inventive steps and capable of industrial
application. If opposition is filed on one or more of the various grounds spelt
out in S.25 which would include novelty, obviousness, lack of inventive steps,
etc. and the documents or evidences are belatedly or improperly produced to
support the case, the Controller cannot shut his eyes and allow an application
merely because the documents have been produced improperly.
On the other hand, the Controller has a duty in law to make sure that an
application should not be granted wrongly to the provisions of the Patents Act.
Because, that which ought to be in public domain would be wrongfully be
granted a monopoly and it is the duty of the Ld. Controller to bring it back to
the public domain in public interest.
Opponent submitted that since question has been raised, Opponent intend to
submit the supplementary affidavits meeting all the legal formalities.
Applicant argued that no expert testimony evidence in support of obviousness
was provided by the Opponent.
Opponent's rebuttal:
It was submitted that:
- Subject matter in dispute is straight forward and the invention as well the
prior art are clearly understood, the Ld. Controller which any way is a
technical person can always act on the documentary evidence and decide the
- There is no need of citing expert evidence in each and every case. Patent
Office in each case before it has to examine the inventive step of an invention
and by definition the aspect of obviousness has also to be taken into account.
- The examination of an application is carried out by a qualified technical
examiner, being a technical person which are wholly guided by the prior art
before them and the knowledge that they possess by way of qualification and
experience and on making themselves familiar with the general common
knowledge related to a specific area of knowledge relevant for a given case.
Therefore, if the Law requires that obviousness cannot be established without
expert testimony then the patent system itself will collapse because every
applicant for patent will require the Controller to prove his objections on
inventive step and obviousness with expert evidence.
- Also, when reference to a person of average skill is made it refers to the
knowledge that a person who has understood the invention would derive from
the prior art as well as the common general knowledge associated with such
- Therefore, on the basis of above it was submitted that, filing of expert
evidence is not a sine qua non to make out a case of obviousness.
- Opponent relied upon following case laws for support which were handed-
out during the hearing. The relevant paragraphs there from are set-out for
ready reference:
POSITION IN EP:
THE BOARD OF APPEAL OF THE EUROPEAN PATENT OFFICE
IN T 0375/00 HELD:
Page 09, last lines of Para 1.2.2 under heading Unnamed Experts:
"In the opinion of the Board an expert is only then necessary when the Board
does not consider itself in a position to decide upon a matter without technical
assistance.
As the Board includes two technically qualified members such cases will be
rare and will only occur in special circumstances. Such special circumstances
do not occur in the present case which is a relatively simple mechanical case.
Moreover, it was open to the appellant for himself to actively find the
necessary evidence. The appellant has not done this. If the Board were to be
active in seeking experts to help the case of a party then the Board could be
open to an accusation of not acting impartially. It is therefore neither
necessary nor desirable for the Board to obtain the evidence of an expert in
this case."
BOARDS OF APPEAL OF THE EUROPEAN PATENT OFFICE IN T
Page 01, Second last paragraph, under "Headnote":
"When evaluating evidence it is necessary to distinguish between a
document which is alleged to be part of the state of the art within the meaning
of Article 54(2) EPC - in the sense that the document itself is alleged to
represent an instance of what has been made available to the public before the
priority date of the opposed patent - and a document which is not itself part of
the state of the art, but which is submitted as evidence of the state of the art or
in substantiation of any other allegation of fact relevant to issues of novelty
and inventive step.
In the first situation, a document is direct evidence of the state of the
art; its status as state of the art cannot normally be challenged except on
authenticity. In the second situation, a document is also evidence albeit
indirect; it provides a basis for an inference about, e.g. the state of the art,
common general knowledge in the art, issues of interpretation or technical
prejudice etc. - an inference which is subject to challenge as to its plausibility.
Only a document of the first kind can be disregarded on the sole ground
that it is post published; documents of the second kind do not stand or fall by
their publication date even on issues of novelty and inventive step."
POSITION IN THE USA:
SAM HOUSTON V. THE POLYMER CORPORATION; 78-2714, 78-
2860; UNITED STATES COURT OF APPEALS, NINTH CIRCUIT:
Last paragraph under heading "OBVIOUSNESS"
Section 103 of 35 U.S.C. establishes nonobviousness as one of three
conditions of patentability. The ultimate question is one of law, but the legal
conclusion is resolved against the background of three factual inquiries: (1)
the scope and content of the prior art; (2) differences between the prior art
and the claims in the suit; (3) the level of skill in the pertinent art. Graham v.
John Deere Co., 383 U.S. 1, 17, 86 S.Ct. 684, 693, 15 L.Ed.2d 545 (1966).
STANDARD OF REVIEW
We begin with the proposition that: When Fed.R.Civ.P. 56 standards are met
and the court, without the aid of expert testimony, can understand the prior
art and patent claims, summary judgment may be proper.
PHILIP W. WYERS AND WYERS PRODUCTS GROUP, INC. V.
MASTER LOCK COMPANY; 2009-1412; UNITED STATES COURT OF
APPEALS FOR THE FEDERAL CIRCUIT:
Page 14, last paragraph:
"The Court also made clear that expert testimony concerning motivation to
combine may be unnecessary and, even if present, will not necessarily create a
genuine issue of material fact.
See id. at 427. We had held that the district court erred in granting summary
judgment, as the affidavits of Teleflex's two experts stating their opinion that
the invention was nonobvious created a material issue of fact. We had noted
that "[a]t the summary judgment stage of a proceeding, it is improper for a
district court to make credibility determinations." Teleflex, 119 F App'x. at
Page 16, Lines 7 onwards
"We furthermore concluded that no expert opinion was required to support
the obviousness determination, because the technology was "easily
understandable." Id. at 1329-30 (quoting Cen-tricut, LLC v. Esab Grosup,
Inc., 390 F.3d 1361, 1369 (Fed. Cir. 2004)); see also Sundance, 550 F.3d at
Page 16, Second paragraph
"Thus, in appropriate cases, the ultimate inference as to the existence of a
motivation to combine references may boil down to a question of "common
sense," appropriate for resolution on summary judgment or JMOL. See
Perfect Web, 587 F.3d at 1330. Other recent cases have confirmed the
appropriateness of this approach."
Threshold in opposition proceedings
Opponent submits that pre-grant opposition can be filed by any person and
need not to be a person interested. The reason for providing such a broader
scope for challenging an application for patent before grant is express in itself
that no unwarranted patent be allowed to grant. Because, that which ought to
be in public domain would wrongfully be granted a monopoly and it is the
duty of the Controller to bring it back to the public domain. Opponent totally
disagreed with the statements made in the "Statement and Evidence" that
threshold in opposition proceedings is high. Opponent states that intentions of
the legislature for creating porous filters like pre-grant opposition, post-grant
opposition is express in itself that no frivolous invention should be allowed to
grant in public interest.
Onus not discharged
Applicant states that Opponent failed to discharge the Onus.
It was submitted that there is no statutory presumption of validity even of a
granted patent in the Act. In fact, there is Section 13 (4) which clearly says
that the examination and investigations required U/S 12 and 13 shall not be
deemed in any way to warrant the validity of the patent.
Therefore, once the Opponent produces his prima facie evidence regarding
novelty, inventive steps and other grounds, the initial onus is discharged by
the opponent and onus shifts to the patentee.
Opponent's Submissions on Admissions made by the Applicant
The Applicant has made several admissions in the "Statement and
Evidence", and also during the oral proceedings namely:
Applicant repeatedly admitted to overcome the insufficiency objections
that, example 1 of the application results in Polymorph-1 of Sorafenib
Tosylate directly.
Opponent's Submissions
Opponent submitted that example 1 of the impugned application forms the
part of prior art, which is admitted by the Applicant too (see page 17, para 34,
In this regard, Applicant place reliance on Section 58 of the Indian Evidence
Act which states that "Facts admitted need not be proved"
Opponent submitted that this statement of Applicant is also supported by the
Affidavits filed the Opponent. Therefore, as admitted by the Applicant and as
shown by the Opponent Polymorph-1 of Sorafenib tosylate is merely a prior
art, hence lacks novelty in view of D1 which discloses Sorafenib tosylate.
Applicant also challenged that, D1 does not refer to any particular parameters
of the Sorafenib tosylate. In this regard Opponent submitted that, it is a well
settled rule that inherent anticipation does not require disclosure of particular
In this regard Opponent relied upon Manual Of Patent Practice And Procedure
2008, pages 25 and 26, section 3.4.7 which states that "a prior art experiment
which, when performed, reliably produced a particular result "more than 99
percent of the occasions on which it is conducted" would be regarded for the
purposes of disclosure as "inevitably" leading to the result in question. It
follows that a claim which defines an invention by reference to parameters,
for example, of a process or a product, is anticipated by a disclosure, which
when put into practice would necessarily fall within the scope of the claim,
even if the disclosure does not refer to these particular parameters"
Applicant in the "Statement and Evidence" clearly states that contents
of representation filed by the Opponent under paragraph 2 or more particularly
under paragraphs 2.2a, 2.2b and 2.2c are misleading (see page 14-15, para 27-
29 of the Applicant's Statement and Evidence filed U/S 25(1)).
Opponent submitted that all the statements made under paragraph 2 of the
representation are referred from the impugned application only. Therefore, as
admitted by the Applicant that these statements are misleading, application
should be rejected prima facie for making frivols or misleading statements in
the application for patent.
Opponent's Submissions on Affidavits Filed by the Applicant
Applicant submitted Affidavit of Dr. Britta Olenic, annexed as
Annexure B at page 42 of the "Statement and Evidence" to show that
Polymorph-II of Sorafenib tosylate is much more sensible to mechanical stress
compared to Polymorph-I.
Opponent submitted to the Ld. Controller that test was not conducted
according to proper standards. It was submitted that there are many flaws in
the test and do not find any place according to law as well as science.
Opponent submitted that polymorph I was grinded only for 30 seconds,
and question remains unanswered that what will happen if said polymorph
would have been grinded for 1 minute to 5 minutes. Opponent submitted that
there is no answer to such questions in the test data.
Opponent submitted that 30 second time duration is a just a spark
moment and should not be considered as a proper test.
Opponent submitted that both the grinding test for polymorph-I and
polymorph-II were conducted at two different dates. There is no answer as to
why the test was not conducted on same date and by the same person.
Opponent submitted that there is no reference to the source of
polymorph-II in this Affidavit. The question remains unanswered as to how
the polymorph-II was prepared (process details).
There is no XRPD and DSC data of the grinded polymorph-I as well
grinded polymorph-II of the Sorafenib tosylate in the affidavit of Dr. Olenic.
There is only reference to untreated forms of Sorafenib tosylate at page 21 of
the impugned application.
No data (XPRD OR DSC) is provided to show partial conversion of
form II to amorphous, as mentioned in the affidavit.
Based on above flaws, Opponent submitted that affidavit of Dr. Britta Olenic
should be rejected in-limine.
Additionally, Applicant submitted Affidavit of Dr. Kerstin Pauli,
annexed as Annexure C at page 53 of the "Statement and Evidence" to show
that dissolution of Polymorph-I of Sorafenib tosylate is higher than its free
Opponent submitted that affidavit does not provide any comparative data
between Polymorph-I and Polymorph-II of Sorafenib tosylate. It is well settled
rule that comparison should be between recent previous prior art and which
D1 in this case which discloses Sorafenib tosylate. Therefore, affidavit of Dr.
Pauli has no value in the instant case.
With regard to the averments made in the affidavit, for all one knows the
higher solubility that is attributed to the polymorph-I of Sorafenib tosylate
may actually be a property of Sorafenib tosylate itself. One does not have to
be an expert in chemistry to know that salts normally have much better
solubility than compounds in free base form.
Therefore, it was submitted that said affidavit does not fulfil any purpose in
the instant case.
Applicant's submission preliminary issues
(A) Reliance placed by the Opponent on Novartis AG v Union o{lndia & Ors.
(MANU SC/0281/2013) -Misplaced:
2. At the very outset, it is submitted that the reliance placed by the Opponent
on case law titled Novartis AG v Union of India & Ors. Civil Appeal nos.
2706-2716 of 2013 which is annexed hereto and marked as Annexure-1 is
misplaced. In this regard, the attention of the Learned Controller is drawn to
paragraph 191 of the said judgement which is reproduced herein below for the
Learned Controller's ease of reference:
We have held that the subject product, tire beta crystalline form of imatinib
Mesylate, does not qualify; the test of Section 3(d) of the Act but that is not to
say that Section 3(d) bars patent protection for all incremental inventions of
chemical and pharmaceutical substances. It will be a grave mistake to read
this judgment to mean that section 3(d) was amended with the intent to undo
the fundamental change brought in the patent regime by deletion of section 5
from the Patent Act. This is not said in this judgment.
It is submitted that from the observation made by the Hon'ble Supreme Court
of India, it is evident that the Hon'ble Court made it clear that the observations
made therein were specific to the subject matter before it and as such, the said
case law cannot be interpreted to mean that it bars patent protection for an
incremental inventions of chemical and pharmaceutical substances. Each case
has to be decided on its own merits and therefore, the observations made
therein will not have the blanket application as has been the understanding of
the Opponent. For the reasons detailed out herein below, it is submitted that
the said case law has no application, whatsoever, to adjudicate upon the issues
involved in the matter.
(B) Grounds of Pre-Grant in the present representation are Frivolous:
3. The Applicant humbly submits that the grounds on which the present
representation has been filed by the Opponent and the argument set forth
therein are frivolous in that they have not fathomed and appreciated the
Applicant's invention, as will be evident in the Applicant's corresponding
submissions to the statement which are enumerated herein below for the
Controller's just consideration.
4. At this stage, it is pertinent to draw the attention of the Learned Controller
that the Opponent in the present has failed to annex the amended and/ or
original set of claims on records. In absence of filing the claims which are
under opposition, it can safely be concluded that the submissions made by the
Opponent in the present representation are based on conjectures and surmises
and are baseless. On this ground alone, the present representation ought to be
(C) No evidence filed by the Opponent:
5. At the very outset, the Applicant strongly objects to the revised declarations
which were handed over to the learned Controller with a copy to the Applicant
at the time of hearing on February 5, 2015, to be taken on record. It is
submitted that at this late juncture no document, whatsoever, can be taken on
record. In fact, the learned Controller had also indicated in the letter intimating
the hearing that arguments will be based on the submissions already made.
6. Without prejudice to what has been stated hereinabove it is submitted that
the declarations (Exhibits 12 to 14), purported to be affidavits filed by the
Opponent, by no stretch of imagination, can be treated as evidence in as much
as they hold no sanctity in the eyes of law. It is a settle proposition of law that
the importance of verification is to test the genuineness and authenticity of
allegations and also to make the deponent responsible for allegations. In
essence, verification is required to enable the court to find out as to whether it
will be safe to act on such affidavit evidence. It is submitted that the
declarations, original or revised, filed by the Opponent are devoid of any such
verification clause. In this regard, the Applicant refers to and relies upon,
Section 3(a), Section 35 of the Indian Stamps Act, 1899, Section 139 read
with Order 19 Rule 3 of the Code of Civil Procedure, 1908 and De1hi High
Court Rules, Chapter 12 (Oaths affirmations and Affidavits) Rule 10, Rule 11
and Rule 15 thereof which is annexed hereto and marked as Annexure*2
(colly). The Applicant in support of the settle proposition mentioned herein
above refers to and relies upon case laws, Amar Singh v Union of India [2011]
7 SCC 69 at page 78-79, paragraphs 22-24, State of Bombay v Purustwttam
Jognayak [1952} SCR 674 at paragraph 15, Sundar Industries v General
Engineer Works [C.R.No. 1165 of 1981] at page 130, Ramas/umker Pathak v
The Controller, Central Excise, Allahabad and Drs. (1970) SCConline All
276 at paragraph 19 which is annexed hereto and marked as Annexure-3
In view of the settled proposition of law, it is evident that the purported
affidavits are not only devoid of any evidentiary value, but also inadmissible
as evidence. In view of the above, it is submitted that the learned Controller
ought not to take cognizance of the said declarations.
7. It is further submitted that Section 77(1)(c) read with Section 79 of the
Patents Act, 1970 (hereinafter referred to as "the Patents Act") mandates that
evidence before the Controller should be in the form of an affidavit. It is also
submitted that the issue as to whether the subject patent application lacks
inventive step or not cannot be decided by making mere submissions which in
either event are based on conjectures and surmises, in a manner as have been
made by the Opponent in the present representation.
8. Without prejudice to the above, it is submitted that it is a settled proposition
of law that in order to assess obviousness, the Court would invariably require
the assistance of expert evidence. In this regard, the Applicant refers to and
relies upon the case titled Molnlycke v Proctor & Gamble. [1994] R.P.C 49 at
page 113 which is annexed hereto and marked as Annexure4 and Strix Limited
P Malmraja Appliance Limited (I.A. No. 7441 of 2008 in CS (OS) No. 1206 of
2008 which is annexed hereto and marked as Annexure-5.
9. In the present case, the Opponent has failed to corroborate the submissions
by placing cogent evidence in the form of an affidavit on record as mandated
by Section 79 of the Patents Act and also being the settled proposition of law.
10. It is submitted that it is a settled proposition of law that the onus as to the
invalidity of a plaintiff's patent and the grounds of insufficiency of description,
want of novelty, absence of inventive steps and want of utility was rightly
placed on the defendants. In this regard, the Applicant refers to and relies
upon the case titled Raj Prakash v Mangat Ram Chowdhry & Ors (R.F.A. (05)
2 of1973) which is annexed hereto and marked as Annexure--6. In view of the
above, the present representation ought to be outrightly rejected.
(D) Threshold of inventive step much higher in Opposition proceedings than
in case of revocation proceedings:
11. It is submitted that the threshold of inventive step is much higher in
Opposition proceedings than in revocation proceedings. In case, the Learned
Controller has an iota of doubt regarding the inventive step, the Learned
Controller should allow the grant leaving the question to be finally decided,
when an occasion arises, by Intellectual Property Appellate Board (IPAB)
and/ or the High Court. In "Patent Law" by P. Narayanan, Fourth edition, the
author has at pages 403 and 213 (which is annexed hereto and marked as
Annexure-7) distinguished the inventive step requirement in case of an
opposition proceeding and in case of a revocation proceeding as under:
"The words "clearly do not involve any inventive step" in Section 25(1)(e)
would appear to indicate that in an opposition proceeding the evidence
required to establish lack of inventive step would be more stringent.''
"Under Section 64(1) (/) obviousness and lack of inventive step is also
mailable as a ground of revocation of a patent by petition before the High
Court. In opposition proceedings under Section 25(1)(e) and Section 25(2)(e)
it must be shown that the invention "clearly" does not involve any inventive
step while there is no such qualification under Section 64(1)(/). This shows
that if the matter is in doubt, the Controller may allow the grant /earring tire
question to be finally decided, when an occasion arises, by the High Court."
(E) Scheme of the Patents Act - The Opponent ought to have put the best case
12. It is submitted that before the Learned Controller adjudicates upon the
present representation by way of an opposition filed by the Opponent against
the grant of patent of the patent application, it is pertinent to draw the attention
of the Learned Controller to the relevant provisions of the Patents Act which
will provide an insight of the scheme of the Patents Act. It is submitted that
prior to the January 1, 2005, there was no concept of post-grant opposition and
Section 25 provided opposition to grant of patent. It is submitted that Section
25(1) of the Patents Act read with Rule 55 of the Patents Rules, 2003
(hereinafter referred to as "the Rules") contemplates representation by way of
an opposition which is a diluted form of representation.
13. Rule 55 of the Rules provides the procedure for pre-grant Opposition
under Section 25(1) of the Act. Rule 55(1) mandates the Opponent to file
statement and evidence, if any, in support of the representation. Rule 55(3)
states that on consideration of the representation, if the Controller is of the
opinion that the application for patent shall be refused or the complete
specification requires amendment, he shall give notice to the Applicant to that
effect along with a copy of such representation, whereupon, the Applicant, as
per Rule 55(4), shall file his statement and evidence, if any, in support of his
application within three months from the date of notice. In view of the above,
it is evident, that what is contemplated for the Applicant under Rule 55 (4) of
the Rules is to file statement and evidence in support of his application and not
reply statement (which is the case re post-grant opposition proceeding i.e.,
reply to written statement filed by the Opponent as contemplated under Rule
58 of the Rules re post-grant opposition proceeding). Accordingly, each of the
parties is required to put its best case in support of its respective submissions.
14. It is further submitted that as the Rules are silent on filing of a rejoinder by
the Opponent in response to the statement and evidence of the Applicant in
support of the application and also on filing of the reply evidence by the
Opponent as contemplated under Rule 59 of the Rules re post-grant
opposition, the Opponent ought to put the best case forward, which, in the
absence of any cogent evidence in the form of an affidavit, as mandated by
Section 79 of the Act, the Opponent has miserably failed.
(F) Ex post (acto analysis- Not permissible:
15. It is submitted that it is a settled proposition that care must be taken not to
construct an argument of obviousness based upon ex post facto analysis.
Hindsight is not to be confused with foresight. Such analysis "is unfair to
inventors". Obviousness is to be considered without knowledge of the
invention otherwise that consideration amounts to impermissible ex post facto
analysis. It is further submitted that obviousness has to be answered not by
looking with the benefit of hindsight at what is known now and what was
known at the priority date and asking whether the former flows naturally and
obviously from latter, but by hypothesising what would have been obvious at
the priority date to a person skilled in the art to which the patent in suit relates,
who is assumed to have access to what was known of the art immediately
before the priority date.
16. It is submitted that it is settled proposition of law that while finding an
answer to the question of whether or not improvement made by the plaintiff is
only a workshop improvement that is so obvious to a person skilled in the art,
one must cautiously remove the hindsight bias. In this regard, the Applicant
refers to and relies upon the case titled British Westinghouse l' Braulik (1910)
27 R.P.C. 209 at 230 which is annexed hereto and marked as Annexure-8 and
M. C. Jayasingh v. Mishra Dhatu Nigam Limited(2014) SCC Online Mad 163
which is annexed hereto and marked as Annexure-9.
(G) Inevitable Results must be strictly proved i.e., beyond reasonable doubt:
17. It is submitted that "inevitable results" must be strictly proved i.e., "must
result" "necessarily result'' in the claimed invention. It is submitted that it is a
settled proposition that even though it was "overwhelmingly likely" that the
prior art had formed a composition within the claims of the patent in suit, this
was not enough for the purposes of anticipation. It is further submitted that
inevitable result objection should only be raised where there "can be no
reasonable doubt as to the practical effect of the prior teaching". In fact, it has
been observed by the Courts that "likelihood, even overwhelming likelihood"
does not suffice in this regard, the Applicant refers to and relies upon the case
titled Ferag l' Muller Martini [2007] EWCA Civ 15 at paragraphs 7 to 9
which is annexed hereto and marked as Annexure-10.
(H}The Opponent has terribly failed to discharge the Onus:
It is submitted that it is a settled proposition of law that in the opposition
proceedings, it is the Opponent, who at the first instance, must plead and
prove that the invention claimed in the patent application does not come
within the purview of "invention" and other criteria as laid down in the Patents
Act. It is only thereafter, that the Applicant is required to counter it.
As evident from the pleadings, the Opponent has terribly failed to make out a
case for refusal for grant of patent inasmuch as the pleadings are vague in
addition to not being supported by evidence in the form of affidavits as
required by the law. It is submitted that the Opponent has also failed to
discharge its onus. Accordingly, the present representation by way of
opposition deserves to be dismissed at the threshold itself.
From the above pleadings, it appears that both the opponents and the applicant
have cited a number of grounds and case laws to establish their stand. Some of
the points are irrelevant/superfluous and some of the points are relevant and
worth discussing in the instant patent application under pre-grant opposition.
As far as the time line and procedural part of the procedure as defined in the
law are concerned, both the opponent and the applicant are well disciplined.
However, the plethora of grounds, prior art documents and case laws put forth
by both the parties are irrelevant in nature need not be addressed. Both the
parties have unnecessarily over burdened the Controller in citing different case
laws. However, I am concerned with the relevant documents, relevant grounds
of opposition and relevant case laws. My decision is based on the outcome of
invention disclosed, analysis of the relevant documents and case laws, and the
argument made by both the opponents and applicant.
Having considered the detailed arguments of both the parties, the teachings of
the various prior art documents on record, the affidavit (s) filed by both the
parties, I shall now deal with each ground of the opposition as discussed
during the hearing.
As far as Preliminary Issues are concerned, all the relevant issues are taken
into consideration while deciding the case.
Regarding the applicants contention that the documents filed by the opponent
cannot be considered as evidence since they have not been filed as an affidavit
as mandated by section 79 of the Patent Act, it is a settled position that lack of
novelty has to be judged only on the basis of prior publication and/ or use,
whereas inventive step has to be looked into on the basis of prior art in
combination with the common general knowledge. Moreover, rule 57 of the
Patents Act requires the filing of the written statement and the facts on which
the opponent makes out his case. The requirement of evidence to be filed is
optional. If the opponent is successful in proving obviousness on the basis of
documents in combination with the common general knowledge, then
additional evidence may not be required. The applicant has rightly pointed out
that the affidavits filed by the opponent II were not in the manner as
prescribed U/s 79 of the Patents Act. But an important fact that needs to be
taken care of is that, as such patent cannot be granted on the grounds that the
documents are belatedly or improperly produced before the Controller. At this
point, it is to be noted that the opponent has submitted properly executed
affidavits with verification clause in accordance with the procedure as
prescribed U/s 79 of the Patents Act, along with hearing submissions.
Considering the fact that patents grants monopoly rights to the applicants &
this right cannot be merely given on the basis of failure to produce the
evidence in the form of affidavits as required under Patents law. Affirming
with the principles of natural justice & in public interest these affidavits are
taken on record. As such the affidavits filed are of not of much significance
or relevance while deciding this matter. IPAB order 173 of 2013 clearly
requires that the opponent has to plead and prove his case. In this regard, the
opponents have pleaded their case on the various documents relied upon by
them in their written statement. Whether these documents relied upon by the
opponent in combination with the common general knowledge will be
adequate to establish their challenge on the ground of obviousness will be
dealt with by me hereinafter.
GROUND 1: CLAIMS NOT PATENTABLE UNDER SECTION 3(c)
After going through the submissions of both the parties, it is clear that the
application relates to a novel form, thermodynamically stable at room
temperature of the tosylate salt of sorafenib which is different from sorafenib
base itself. The document WO 00/42012 relates Sorafenib base & does not
describe any polymorphic form of sorafenib tosylate salt. Though the
documents WO 03/047579 and WO 03/068228 describe a tosylate salt of
sorafenib but as such there is no mention of any polymorphic form. In absence
of any evidence that any polymorphs described in the application were known
& that the polymorph 1 would be inherently produced, it cannot be concluded
that the inventions is a mere discovery of a scientific principle & thus does not
fall under the ambit of Section 3(c) of the patents Act.
I conclude that such a ground of opposition is not validly established by the
opponent I.
GROUND 2: SECTION 25(1) (g): INSUFFICIENT DISCLOSURE
That the complete specification does not sufficiently & clearly describe
the invention or the method by which it is to be performed.
After going through the arguments & submissions of both the parties on the
above mentioned ground where section 10(4) is in question, I am of the
opinion that the complete specification of the patent application sufficiently
and clearly describes the invention as well as the method by which it is to be
performed. The polymorphic forms of sorafenib tosylate are sufficiently and
clearly described in the description in tables 2 to 6 by X-ray diffractometry, IR
spectroscopy, Raman spectroscopy, FIR spectroscopy and NIR spectroscopy.
The polymorph of example 1, i.e., polymorph ll, is not part of the invention
claimed in the patent application. The fact as pointed out by applicant that
over 40 patents have been granted on corresponding applications filed in
various countries where the complete specification fulfilled the test of
describing the invention sufficiently and the best mode of carrying out the
invention further supports the fact that the complete specification clearly
fulfils the requirement of Section 10(4) of the Patents Act by sufficiently &
clearly defining the invention or the method by which it is performed.
I conclude that such a ground of opposition is not validly established by the
opponents.
GROUND 3: INVENTION NOT PATENTABLE UNDER SECTION 3(i)
In view of deletion of claims 5 to 6 and 9 to 11, which were related to the use
of the compound of claim 1 or a pharmaceutical composition thereof for the
treatment of certain disorders or diseases during reply to First Examination
Report dated 04th March, 2014.
The opposition ground is rendered moot.
GROUND 4: CLAIMS NOT PATENTABLE UNDER SECTION 3(e)
Section 3(e) the Patents Act, 1970 mandates that, for the claims relating to the composition or combination, the claims should define all the novel, inventive features in terms of its percentage or ratio of the constituents used to prepare the composition or combination clearly, ensuring that the composition or combination is not just a mere admixture but also a synergistic composition supported with working examples.
It is also a fact that, when the claims of a invention relates to any product or compound, it is the normal practice of the inventors to incorporate claims relating to composition or combination in order to broaden the scope of the claims, so no one infringes the product claims & in such a case there is as such no need to incorporate any working examples for preparation of such composition or combination in the patent application, subject to the condition that the product or compound qualifies for a invention under section 2(1)(j) of the Patents Act , 1970 i.e. the product is novel, inventive & has industrial applicability .
The question of Section 3(e) of the Patents Act, 1970, comes in picture only when the product or compound qualifies the test for an invention under section 2(1) (j) of the Patents Act, 1970
In the present case it the clear that though invention clears novelty & industrial applicability tests but fails to qualify for an invention under Inventive step.
Since the claims lack inventive step on the basis of cited documents, the composition or combination claims does not have any relevance & are not patentable under Section 3(e) of the Patents Act, 1970.
I conclude that such a ground of opposition is validly established by both the opponents.
GROUND 5: CLAIMS NOT PATENTABLE AS LACKING NOVELTY
UNDER SECTION 25(1) (b)/(c)
After going through the submissions, the documents acknowledged in the
specification & cited by the opponent I, it is clear that Document WO
00/42012 does not describe any polymorphic form of sorafenib tosylate salt.
WO 03/047579 and WO 03/068228 describe a tosylate salt of sorafenib but
without any specification of the polymorphic form. As regards Scott Wilhelm
et al also narrates the use of the compound BA43-9006 in clinical settings- the
same constitutes a specific use of the compound BA43-9006.
None of the document provide a more thermodynamically or mechanically
stable polymorphic form of sorafenib tosylate salt. The opponents' argument
that in absence of any data it must be presumed that the cited document
disclosed the polymorph 1 of the applicant is not found to be persuasive
Also after going through the submissions, the documents acknowledged in the
specification & cited by the opponent II.Though the experiments were carried
out by the method as disclosed in the specification or from the known method
it must be appreciated that it was the applicant who has carried out the at first
instance. The applicants explanation to the opponents assertion that the
opponents could not reproduce the polymorph II while conducting the process
of claim 1 in the affidavit (submitted with hearing submission) might be due
to unintentional seeding as supported by the applicant in Annexure A cannot
be denied. Also just relying upon the theory of disappearing polymorphs
which exists or not & the mere assertion that polymorph–I must have formed
in view Document D1 is not a concrete proof to establish novelty.
The opponents' reliance on document D3 that modification -3 of sorafenib
tosylate was used in the clinical studies & D4 that a tablet formulation of
sorafenib tosylate was used in clinical studies & according to the common
knowledge that "compounds in most stable form are required for
efficacious and stable drug formulation" (refer to Page 12, para 21, line 05-
06 of the "Statement and Evidence") & then drawing inference that a any
composition of Sorafenib tosylate would certainly use stable form which is as
per Applicant's statement is polymorph–I may be logic that may be acceptable
but does not help in establishing novelty, thus claim 1 is novel.
Since claim 1 novel claims 5-7 & claims 10-13 are also novel.
At this juncture I would rely on the decision by Hon'ble IPAB, In
OA/8/2009/PT/CH (250/2012) rejected novelty ground "to defeat novelty, the
appellant should show that an earlier document, disclosed all that the
patentee is seeking to patent. And that each limitation of the claimed invention
is found in a single prior art reference. The appellant has not done this. So the
ground of novelty is rejected. In the present case both the Opponents have
relied on more than one document to bring out their case but were still
I conclude that such a ground of opposition is not validly established by both
the opponents.
GROUND 6: PRIOR USE (SECTION 25(1) (d)
Section 25(1) (d) mandates the opponent to show that the invention so far as
claimed in any claim of the complete specification should have been publicly
known or publicly used in India before the priority date of the claim. The
explanation that follows to it clearly specifies
Explanation – For the purposes of this clause, an invention relating to a
process for which a patent is claimed shall be deemed to have been publicly
known or publicly used in India before the priority date of the claim if a
product made by that process had already been imported into India before
that date except where such importation has been for the purpose of
reasonable trial or experiment only.
Opponent I asserts that the sorafenib tosylate is mentioned in WO 03/047579
and WO 03/068228, but the documents nowhere discloses any polymorphic
form I of sorafenib tosylate. The other document relied i.e. Scott Wilhelm.et
al. "Current Pharmaceutical Design 2002" [referred to as Annexure E in the
Opponent's statement] discloses only the use of the compound BAY 43-9006
which is sorafenib itself & does disclose polymorph I of sorafenib tosylate.
Thus the opponent I fail to provide any concrete evidence to support his
claims that polymorphic form I of sorafenib was publicly known or publicly
used in India before the priority date of the claim tosylate.
The opponent II relied on the post published documents D3 & D4.
D3 which is a scientific discussion of the pharmacological activity of
sorafenib in treating cancer, published by the EMEA in 2006, i.e. after the
filing date of the present patent application discloses reports on two clinical
studies 100391 (phase II study) and 11213 (phase III study), allegedly using
film-coated tablets containing 274 mg of sorafenib tosylate, microcrystalline
cellulose,croscarmellose, hypromellose sodium lauryl sulphate, magnesium
stearate, water, titanium dioxide and red ferric oxide (03, page 2149, last
Though D3 discloses that the active substance exhibits polymorphism and
crystallizes in three different modifications (modification I II and III) (D3,
page 3/49, first paragraph), but it fails to provide that which of the
modifications have been used for the clinical studies (page 29/49, second
paragraph and page 36/ 49).
Document D4 is an approval letter by FDA. It only discloses that the studies
were carried out between September 25, 2002 and September 2006 (study
100391) and between November 19, 2003 and September 2006 (study 11213)
(see e.g. D4, page 3, item 5).
It is clear that D4 mentions the date when the clinical studies started, but there
is no clear cut evidence of the date of delivery of the sorafenib tosylate tablets
& its use in public domain before the priority date of the present patent
Both the documents fails to disclose that sorafenib tosylate in the form of
polymorphic form I has been used in the clinical trials. It is evident from the
documents submitted that the clinical studies were not conducted in the public
domain, but were subject to a specific confidentiality.
Thus it is clear from the arguments & submissions that the opponents rely
only on the post published documents & confidential clinical trials which are
not in public domain for free use.
None of the prior art mentions the use any polymorphic form of sorafenib
tosylate, nor does any concrete evidence provided by the opponent for prior
use of the polymorphic form I of sorafenib tosylate. Therefore the ground of
opposition U/s 25(1) (d) is not sustained.
I conclude that such a ground of opposition is not validly established by both
the opponents.
GROUND 7: INVENTIVE STEP (SECTION 25(2) (e)
The applicant states that the present invention relates to a novel form,
thermodynamically stable at room temperature, of the tosylate salt of 4-{4-
[({[4-chloro-3-(trifluoromethyl) phenyl] amino} carbonyl) amino] phenoxy}-
N-methylpyridine-2-carboxamide, to processes for its preparation, to
medicaments comprising it and to its use in the control of disorders like
cancer. The problem associated with the pharmaceutical products is to have
always the same constant properties to guarantee constant and reliable
efficacy. From the applicants' statement, it is clear that there is a need to find
the most stable form of a compound because only the most stable form can
ensure that all properties and characteristics regarding stability, dissolution
rate, shelf life, efficacy, and bioavailability remain constant during
manufacturing, storage, and administration. The applicant addresses the
problem by providing a polymorphic form I of sorafenib tosylate which is
surprisingly more stable than the other polymorphs found and ensures a
constant & reliable efficacy, therapeutic or otherwise.
From all the documents cited & disclosures & evidences filed the teachings
that flow from them is as follows,
It is evident from the description and claims of the present invention relates to
a novel form, thermodynamically stable at room temperature, of the tosylate
salt of 4-{4-[({[4-chloro-3-(trifluoromethyl) phenyl] amino} carbonyl) amino]
phenoxy}-N-methylpyridine-2-carboxamide, to processes for its preparation,
to medicaments comprising it and to its use in the control of disorders like
The tosylate salt of 4-{4-[({[4-chloro-3-(trifluoromethyl) phenyl] amino}
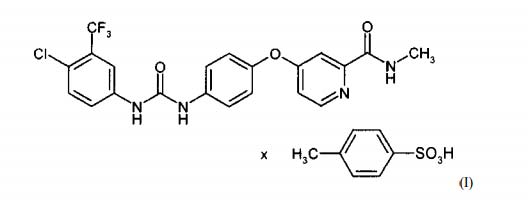
carbonyl) amino] phenoxy}-N- methylpyridine-2-carboxamide is mentioned
in WO 03/068228 and WO 03/047579 and corresponds to the compound of
the formula (I):
The compound 4-{4-[({[4-chloro-3-(trifluoromethyl) phenyl] amino}
carbonyl) amino] phenoxy}-N-methylpyridine-2-carboxamide is described in
WO 00/42012 and corresponds to the compound of the formula (11):
The compounds and their salts, disclosed in WO 00/42012, for example
tosylates, are described there as inhibitors of the enzyme Raf kinase and may
be used for the treatment of disorders, for example cancer.
Conclusions for Opponent I
After careful consideration of the arguments of both the parties on the
documents cited it is clear that Annexure "A" i.e. WO 0042012 discloses the
base Sorafenib. The compounds and their salts, disclosed in WO 00/42012, for
example tosylates, are described there as inhibitors of the enzyme Raf kinase
and may be used for the treatment of disorders, for example cancer.
Annexure "F" i.e.W0/1996/027592 discloses a process for the preparation of
polymorphic B form of (E)-4- [[3-[2- (4-cyclobutyl-2-thiazolyl) ethenyl]
phenyl] amino]-2, 2-diethyl-4-oxobutanoic acid by agitating Polymorphic
Form A in a solvent and adding the seed crystals of Form B.
Annexure "G" i.e. W0/1999/001444 in example 23 discloses a process for
the preparation of thermodynamically stable polymorphic form I of the
tachykinin receptor antagonist 2-(r)-(1-(r) -(3,5-bis(trifluoromethyl)
phenyl)ethoxy)-3-(s)-(4-fluoro) phenyl-4-(3-5 (-oxo-1hAh-1,2A,-triazolo)
methylmorpholine by effecting the compound of Form II in methanol and
seeded with crystals of Polymorphic form I.
Annexure "H" i.e. A chapter from the book "Advanced Pharmaceutical
Solids" by Jens T. Carstensen, published by Marcel Dekker Inc, 2001. It
clearly discloses the various experimentation techniques to obtain polymorphs
and to convert metastable polymorphs into stable polymorphs. Thus the
process and methods used for making polymorphs or converting a metastable
polymorph into a stable polymorph is well documented in literature.
The teachings that flow from the above cited prior arts is that one can easily
convert one polymorphic form to other polymorphic form & is conversion is
spontaneous. Also the use inert solvent & seeding technology is known from
the prior art for the conversion one polymorphic form to another polymorphic
form. Thus applying the same teachings the process as claimed in the claims
2-4 of the instant invention can be arrived.
Claims 2-4 and claims 8-9 of the impugned patent application deal with
process for the preparation of polymorphic form I of sorafenib tosylate.
Claims 8-9 deal with the process of converting from polymorph II to
Certainly combining the above teachings of the prior art for a skilled artisan,
it is clear cut motivation to prepare stable polymorphic form of Sorafenib
tosylate as claimed in the impugned patent with enhanced properties with
reasonable success by combining the above teachings of any of the references
as mentioned in the cited documents no inventive step resides in the same.
Conclusions for Opponent II
After going through the submissions of both the parties it is clear that
Document D1 (WO 03/068228) as cited by the opponent 2, also disclosed in
the impugned patent specification discloses tosylate salt of sorafenib and its
use in the treatment of disorders in which angiogenesis plays an important role
for example in tumor growth. The teaching that flows from this document is
that tosylate salt of sorafenib is known.
D5 as cited by the opponent is research article published in Chemistry &
industry, 1989, pages 527-529 is a general article which discloses the
common general knowledge in the field of polymorphism surely discloses
that "The thermodynamically stable polymorph needs to be identified. If the
compound is enantiotropic, there will be two or more stable polymorphs and
transition temperatures as well. These can be identified by simple
techniques, for example by stirring or shaking excess solid with solid at
different temperatures. "(See on page 528, column 02). The same article also
on page 527, column 02 under section "Crystals and Crystallisation", "The
use of seed crystals can be helpful in obtaining a desired polymorph.
Manufacturing processes seem to be worked out by trial and error aided by
serendipity, and then adhered to rigidity. "
The teaching that certainly flows from this article clearly suggests that stirring
or shaking and seeding technology is very well known in the art prior can be
used to prepare a thermodynamically stable polymorph.
Thus a person skilled in the art can easily combine the teachings D1& D5 to
arrive at thermodynamically stable polymorph 1 of sorafenib tosylate.
Therefore claim 1 lacks inventiveness.
Claims 2, 3, 4, 8 and 9 relates to the conversion of one polymorph to another
thermodynamically stable polymorph, particularly a process for the
conversion of polymorph-II to polymorph-I.
The disclosure on page 15 & 16 on the specification of the impugned
invention discloses the process for the conversion of polymorph-II to
polymorph-I of Sorafenib tosylate; "Preference is given to preparing the
compound of the formula (I) in the polymorph I by effecting the compound of
the formula (I) in the polymorph (1l) obtained as described in example 1, in
methanol, ethanol, a mixture of both solvents or a mixture of both solvents
with water, preferably a 1:1 mixture with water, and shaking or stirring at a
temperature of from 50°C up to the reflux temperature of the
Solvent, preferably at from 60 to 80°C, in the absence of crystals of a solvate
of the compound of the formula (I), for example in the absence of crystals of
the methanol solvate or the ethanol solvate of the compound of formula (I),for
up to one day. The crystals are cooled to from -30°C to room temperature,
preferably from -25 to 1 0°C, isolated and dried The compound of the formula
(I) is thus obtained in the polymorph I Most preferably isopropanol, ethyl
acetate or a mixture thereof is used as solvent.
"Preference is likewise given to preparing the compound of the formula (I) in
the polymorph I by effecting the compound of the formula (I) in the polymorph
II, obtained as described in example 1, in methanol, ethanol, a mixture of both
solvents or a mixture of both solvents with water, and shaking or stirring at a
temperature of from 1 0°C up to the reflux temperature of the solvent,
preferably at room temperature, for up to 1 day. The mixture is subsequently
seeded with crystals of the compound of the formula (I) in the polymorph I and
stirred or shaken, for example at room temperature, for from 1 hour to 14
days, preferably from 2 hours to 7 days. The crystals are isolated and dried.
The compound of the formula (1) is thus obtained in the polymorph I most
preferably isopropanol, ethyl acetate or a mixture thereof is used as solvent"
Thus from the disclosures Dl in view of D5 it is clear that, polymorph-I of
Sorafenib tosylate is obtained by heating the polymorph-II of Sorafenib
tosylate in an inert solvent. Subsequently, it mentions that seeding is preferred
for obtaining the polymorph-I of Sorafenib tosylate.
Also referring to the disclosure page 02, first paragraph of the impugned
application & the admittance of the fact that Sorafenib tosylate of Dl exist in
polymorph-II, starting from Dl conversion of a known compound to another
thermodynamically stable polymorph certainly is obvious in view of D5.
Therefore the process as claimed in the claims 2, 3, 4, 8 and 9 of impugned
application involves no inventive step of any sort and does not satisfy the
criteria of obviousness.
Document D6 relates to review article published Pharmaceutical Research,
vol. 12, No. 7, pages 1995, 945-954 provides a review of strategic approaches
to remove much of uncertainty by presenting concepts and ideas in the form of
flow charts to control the crystal form (polymorph) of drug substance. D6
provides a review of strategic approaches to remove much of uncertainty by
presenting concepts and ideas in the form of flow charts to control the crystal
form (polymorph) of drug substance.
It outlines investigations of the formation of polymorphs and the controls
needed to ensure the integrity of the drug substance containing either a single
or mixture of polymorphs. D6 on page 946, under the heading; "Formation of
Polymorphs - Have Polymorphs Been Discovered?"The first step in the
polymorph decision tree is to crystallize the substance from a number of
different solvents in order to answer the question: Are polymorphs possible?
Solvents should include those used in the final crystallization steps and those
used during formulation and processing and may also include water,
methanol, ethanol, propanal, isopropanol, acetone, acetonitrile, ethyl acetate,
hexane and mixtures if appropriate. New crystal forms can often be obtained
by cooling hot saturated solutions or partly evaporating clear saturated
solutions. . "
The teaching that flow from this document D6 is the use of specific solvents
during preparation of polymorphs & specifically mentions the use of solvents
such as water, methanol, ethanol, propanal, isopropanol, acetone, acetonitrile,
ethyl acetate, hexane and mixtures. Here I see that same solvents has been
used by the applicants in preparing the polymorph I of Sorafenib tosylate in
the working examples of the impugned application
Thus by combining teachings of D5 & D6, a skilled artisan it is practically
possible to arrive at a process of converting one polymorph to another
polymorph using the solvents mentioned in the D6. Thus process claims lack
inventive merit.
In order to establish the ground of Inventive step as mandated under section
2(1) (j) of the Patent Act, the applicant had asserted that the polymorph
possess unexpected properties. Even if the assertion is considered the claiming
of a polymorph I from polymorph II by using the method which is inherently
disclosed in the prior art does not necessarily make the claim inventive.
However it is crystal clear that the applicant has emphasized more on
physical stability of the polymorph I during preparation, which alone cannot
be considered as a sole factor for technical advancement of the present
invention under section 2(1) (j) of the Patent Act. Thus from the above facts,
it is clear that applicant have failed to establish any technical advancement or
any economic significance of the Polymorph I of sorafenib tosylate over the
disclosures of prior art.
Also the position itself is clear in the landmark judgment which has been aptly
applied for judging Inventive step. I rely on the landmark judgment, which
goes as In Bishwanath Prasad Radhey Shyam Appellant v Hindustan Metal
Industries the Supreme Court of India laid down the importance of assessing
inventive step, as follows:
"It is important that in order to be patentable an improvement on something
known before or a combination of different matters already known, should be
something more than a mere workshop improvement; and must independently
satisfy the test of invention or an 'inventive step'. To be patentable the
improvement or the combination must produce a new result, or a new article
or a better or cheaper article than before. The combination of old known
integers may be so combined that by their working interrelation they produce
a new process or improved result. Mere collection of more than one integers
or things, not involving the exercise of any inventive faculty, does not qualify
for the grant of a patent."
From the aforesaid, I am of the opinion that the compound Sorafenib base &
tosylates salt of sorafenib are also known in the art. The requirement of
finding the most stable form of a tosylate salt to ensure that all properties and
characteristics regarding stability, dissolution rate, shelf life, efficacy, and
bioavailability remain constant during manufacturing, storage, and
administration can be easily achieved by using the above mention cited art and
there is no inventive contribution of the applicant atleast in this arena. Now,
taking up the question of whether identifying the suitable polymorph I from
the known sorafenib tosylate having more stability what contribution it exerts,
I see from the various documents that there is a disclosure of the tosylate salt
also being stable. Now, while judging obviousness any factor that will lead to
reasonable expectation of success is relevant and a person skilled in the art has
a sound knowledge in this field and not completely ignorant. This being the
opinion of the Hon'ble IPAB, I am convinced that having known the
therapeutic activity of sorafenib base & that of sorafenib tosylate, the
requirement of identifying a stable polymorph having specific stability,
dissolution rate, shelf life, efficacy, and bioavailability that remains constant
during manufacturing, storage, and administration identifying the suitable
form which will exhibit these characteristics will be routine experimentation
and cannot be considered as inventive. The position of the applicant that non-
analogous prior art is irrelevant while judging obviousness is incorrect since
all knowledge before the priority date of the patent which is not specific to this
field will be held to constitute common general knowledge. Regarding, the
composition claim & combination claims as such the product & process
claims not inventive, the same are obvious on the face of the prior art. Thus
the claimed invention falls under the ambit of Section 2(1)(ja) in view of lack
of inventive step in absence of any technical advancement over the prior art &
also in the arena of economic significance or both and is obvious for a person
skilled in the art. Thus the invention as claimed in the claim 1 & its dependent
claims lack inventive step & is obvious to a person skilled in the art.
I conclude that such a ground of opposition is validly established by both the
opponents.
GROUND 8: CLAIMS NOT PATENTABLE UNDER SECTION 3(d)
After going to the arguments of both the parties, I am of the opinion that for
a polymorph to qualify for an invention under section 3(d) of the Indian
Patents Act, has to show significant improvement of t h e r a p e u t i c efficacy
as compared to known form. The impugned patent relates to a novel form,
thermodynamically stable at room temperature, of the tosylate salt of
sorafenib, to processes for its preparation, to medicaments comprising it and
to its use in the control of disorders like cancer however there is no disclosure
in the specification as to enhancement in the therapeutic efficacy as compared
to its structurally similar forms i.e. the tosylate salt of sorafenib mentioned in
documents WO 03/068228 and WO 03/047579 & also admitted in the
specification of the applicant. The specification of the impugned application
focuses on physical attributes such as the properties and advantages of a
thermodynamically stable form of Sorafenib tosylate salt. The inventive
feature being claimed as the polymorphic form 1, which is stable enough and
does not convert into another polymorphic form and prevents changes in
solubility or bioavailability profile. There is however no mention in the entire
specification that the polymorph 1 of Sorafenib tosylate salt exhibits better
therapeutic efficacy and treats/contributes to an improvement in the treatment
of cancer. No research data or clinical trials results are disclosed in the
specification nor provided in any form to support therapeutic efficacy.
Further the applicant has relied on the technical affidavit deposed by Dr.
Kerstin Pauli. However after going through it the data as submitted reveals
that the efficacy of the tosylate salt of sorafenib in polymorphic form l is
higher than its base sorafenib due t o a higher d is s ol ut i on in tablets
containing both drugs each.
A simple comparative test for studying the dissolution rate has been conducted
on the polymorph I of sorafenib tosylate & sorafenib base rather than
sorafenib tosylate polymorph II being the closest prior art.
The other affidavit filed Dr. Olenik focuses on mechanical stress test &
concludes that the polymorph I of sorafenib tosylate is more stable &
technically advanced than the other polymorphs including polymorph II &
ensures a constant & reliable efficacy.
Also the problem as addressed by the applicant is to provide a form o f
sorafenib tosylate which has
superior properties regarding
manufacturing, storage, and administration & the solution proposed
is in finding the most stable form of a compound because only the most
stable form can ensure that all properties and characteristics regarding
stability, dissolution rate, shelf life, and bioavailability remain constant
during manufacturing, storage, and administration. Since these factors
will have influence on the bioavailability of the drug rather than the
therapeutic efficacy of the drug. The physical stability of the compound
during formulation cannot be considered as a sole factor for improvement of
therapeutic efficacy of the drug under as required under section 3 (d) of the
Indian Patent Act, almost the same view was expressed in the landmark
decision issued by Hon'ble Supreme court in Novartis case (referring to
paragraphs 180-192) where the Supreme Court was concerned with the
patentability of polymorphic form of imatinib mesylate. It was contented by
the Applicant/Petitioner, Novartis before the Supreme Court that the crystal
form has better physical properties including that it is thermodynamically
more stable, less hydroscopic and has lower hygroscopicity (page 63, para
168). This was supported by expert evidence submitted by the Appellant-
Novartis. Comparison was made of these physical properties of the crystal
form with the base and it was contended that because the crystal form exhibits
better solubility, the same should be taken as a measure of increased
bioavailability and hence, improved therapeutic efficacy. It was contended that
the crystal showed 30% improvement in bioavailability as compared to the
free base (para 168-169, page 63). The Supreme Court considered these
submissions in paragraphs 175-190 and at paragraph 180, especially paragraph
187 the Supreme Court has held that "In whatever way therapeutic efficacy
may be interpreted, this much absolutely clear that the physic-chemical
properties of beta crystalline form of Imatinib Mesylate, namely (i) more
beneficial flow properties, (ii) better thermodynamic stability, and (iii) lower
hygroscopicity, may be otherwise beneficial but these properties cannot even
be taken into account for the purpose of the test of Section 3(d) of the Act,
since these properties have nothing to do with therapeutic efficacy". The
Supreme Court has also held in paragraph 188 that "Bioavailability falls
outside the area of efficacy in case of a medicine…." Further, in paragraph
189, the Court held that "… the position that emerges is that just increased
bioavailability alone may not necessarily lead to an enhancement of
therapeutic efficacy…." The Supreme Court has also held that "… whether or
not an increased in bioavailability leads to enhancement of therapeutic
efficacy in any given case must be specifically claimed and established by
research data…."
Thus the Hon'ble Supreme Court outrightly rejected that the mere comparison
of base with a claimed form as sufficient for establishing therapeutic efficacy
and held that therapeutic efficacy must be established by submitting
appropriate research and clinical data.
In the present context, even if the claim of the applicant is taken into
consideration, it does not have a legal standing in view of absence of any
clinical trials results demonstrating the fact that the newly formed polymorph
1 of sorafenib tosylate is more efficacious than polymorph II of sorafenib
tosylate in terms of therapeutic effects.
Here, I see that all the data furnished by the applicant pertains to physical
attributes such as storage, stability, bioavailability studies resulting in a
thermodynamically stable at room temperature polymeric form of the tosylate
salt of sorafenib, but the requirement of showing enhanced therapeutic
efficacy still remains unaddressed.
The Hon'ble Supreme court has held in the Novartis case that Para 173. The
aforesaid properties, (physical attributes according to Manley), would give
the subject product improved processability and better and longer storability
but, as we shall see presently, on the basis of those properties alone, the beta
crystalline form of Imatinib Mesylate certainly cannot be said to possess
enhanced efficacy over Imatinib Mesylate, the known substance immediately
preceding it, within the meaning of section 3(d) of the Act.
Accordingly, I opine that the invention fails to demonstrate therapeutic
efficacy and therefore fails to fulfill the requirement of a patentable invention
u/s 3(d) of the patents Act.
I conclude that such a ground of opposition is validly established by both the
opponents.
GROUND 9: U/s 25(1) (h), THE FAILURE TO DISCLOSE DETAILS
OF CORRESPONDING FOREIGN APPLICATIONS;
Section 8(1) mandates the applicant to provide information and undertaking
relating to foreign filings within the prescribed period. Rule 12 provides a
period of six months to provide all the details of foreign filings. Section 8(2)
also casts a duty on the applicant to provide information to the controller as
and when required relating to the processing of the application in a country
The applicant has filed first form 3 on 14th March, 2007 disclosing only details
of two countries. The applicant has then updated the details of foreign filings
by filing another form 3 on 14th August, 2012, disclosing the details of foreign
filings in almost 77 countries & that to not in prescribed manner.
Consequently 3 form 3 giving updated details of foreign filings were filed
details that also not in prescribed manner.
In the present case the agent of the applicant has filed second form 3 after a
gap period of five years & only after the opponent one filed his opposition
opposing it under section 8 i.e. after 14th October 2011.
After contesting the ground under Section 8 during hearing & after validly
establishing the ground by opponent, the applicant filed a petition under rule
137 on 19th January, 2015 for obviating the irregularity citing the details were
not available at the time filing. An important point is that ignorance of fact is
an excuse but ignorance of law cannot be pleaded as an excuse. Here the
petition under rule 137 is not taken on record as it is beyond the reasonable
time i.e. after 7 years & is also detriment to the interest of opponent I, as it
was one of the validly established grounds of opposition.
Also compliance under section 8(1) is a continuing one, that is, the applicant
is required to disclose details at regular intervals so as to keep the Patent
Office abreast in prescribed manner. The scheme of law requires that the
Patent Office is kept aware of any foreign filing within six months of such
filing. Hence, rule 12(2) dictates the six month period within which the
Applicant-Patentee has to discharge its duty.
The Applicant has creatively attempted to escape liability under section 8(1)
by furnishing the required details at the last moment & filing a petition for
obviation of delay, thereby, diluting the express provision of law as well as
the object of the law. Hence, one time stray filing cannot be held to have
satisfied the requirement of section 8(1). Further, such a conduct o f t h e
a p p l i c a n t i s n o t permitted so as to dilute the requirements under section 8.
I hereby rely on the orders of Hon'ble IPAB, which has in series of decisions
issued strict guidance that the requirement under section 8 cannot be diluted
by the Ld. Controller (Ajanta Pharma v. Allergan Inc., Order 173/2013; Tata
Chemicals v. Hindustan Unilever, Order 166/2012; Fresenius Kabi Oncology
v. Glaxo Group Order 161/2013).
In the present case the applicant has failed to discharges his duties miserably
under section 8(1) of the Patents Act. Thus the opponent has successfully
established the ground of opposition as contested under section 8.
I conclude that such a ground of opposition is validly established by the
opponent I.
After having considered all the circumstance of this case, representation and
expert evidence of opponents, reply of the applicants, expert evidence in
support of the applicant, written submissions and arguments in the hearing
made by both parties and also my discussion and findings as mention above, I
am of the opinion that the opponents have succeeded in proving their grounds
relied upon by them. The application is refused on the ground of lack of
Inventive step(Section 2(1)(ja), Section 3(d), Section 3(e) & finally Section
25(1)(h) of The Patents Act, 1970. In view of the above, I accept the
representation and refuse to proceed with the application for grant and
therefore, there shall be no patent in pursuance of this application. There is no
order as to costs.
Dated, the 24th day of February, 2015.
(Dr. Ajay S. Thakur)
Assistant Controller of Patents & Designs.
1) M/s Ms. Sanjay Kumar,
Perfexio Legal, Attorneys-at-Law,
9655, Sector-C, Pocket-9, Vasant Kunj,
New-Delhi-110070, India.
2) Dr.Chitra Anand,
Rajeshwari & Associates,
S-208, Lower Ground Floor,
Near ING Vysya Bank,
Panchsheel Park, New-Delhi -110017.
3) Dr. Prachi Tiwari,
Fresenius Kabi Oncology Ltd,
Som Datt Chambers-I, Bhikaji Cama Place,
New-Delhi-110066, India.
Source: http://ipindiaservices.gov.in/decision/1960-DELNP-2007-9548/1960%20order.pdf
REPOBLIKAN'I MADAGASIKARA Tanindrazana – Fahafahana – Fandrosoana COMMUNE URBAINE NOSY BE DISTRICT NOSY BE PROVINCE ANTSIRANANA I – Monographie b. De 1840 à 1896 c. De 1896 à 1960 d. De 1960 à nos jours 2. Situation géographique et délimitation administrative a. Situation géographique b. Organisation administrative 3. Les acteurs de développement
"PROTEZIONE CIVILE EDUCATIONAL" - www.casaleinforma.it/pcivile V° Contingente CRI at the Italian Red Cross Hospital in Baghdad September – October 2003 PROTOCOL FOR THE TREATMENT OF BURNS AT THE ITALIAN RED CROSS HOSPITAL IN BAGHDAD Document drawn up by Dr. Sandro Gregorio doctor at the Orthopaedic and Trauma O.U at the "G. Gaslini" Institute in Genova.






