
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Itp.w3.kanazawa-u.ac.jp
Progesterone and Mifepristone Regulate L-Type Amino Acid Transporter 2 and
4F2 Heavy Chain Expression in Uterine Leiomyoma Cells
Xia Luo, Ping Yin, Scott Reierstad, Hiroshi Ishikawa, Zhihong Lin, Mary Ellen Pavone, Hong Zhao, Erica E. Marsh
and Serdar E. Bulun
J. Clin. Endocrinol. Metab. 2009 94:4533-4539 originally published online Oct 6, 2009; , doi: 10.1210/jc.2009-1286
To subscribe to Journal of Clinical Endocrinology & Metabolism or any of the other journals published by The Endocrine
Society please go to: http://jcem.endojournals.org//subscriptions/
Copyright The Endocrine Society. All rights reserved. Print ISSN: 0021-972X. Online
Progesterone and Mifepristone Regulate L-Type
Amino Acid Transporter 2 and 4F2 Heavy Chain
Expression in Uterine Leiomyoma Cells
Xia Luo, Ping Yin, Scott Reierstad, Hiroshi Ishikawa, Zhihong Lin,Mary Ellen Pavone, Hong Zhao, Erica E. Marsh, and Serdar E. Bulun
Division of Reproductive Biology Research (X.L., P.Y., S.R., H.I., Z.L., M.E.P., H.Z., E.E.M., S.E.B.),Department of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University,Chicago, Illinois 60611; and Department of Obstetrics and Gynecology (X.L.), Qilu Hospital, ShandongUniversity, Jinan, Shandong 250012, People's Republic of China
Context: Progesterone and its receptor (PR) play key roles in uterine leiomyoma growth. Previously,
using chromatin immunoprecipitation-based cloning, we uncovered L-type amino acid transporter
2 (LAT2) as a novel PR target gene. LAT2 forms heterodimeric complexes with 4F2 heavy chain
(4F2hc), a single transmembrane domain protein essential for LAT2 to exert its function in the
plasma membrane. Until now, little is known about the roles of LAT2/4F2hc in the regulation of the
growth of human uterine leiomyoma.
Objective: The aim of the study is to investigate the regulation of LAT2 and 4F2hc by progesterone
and the antiprogestin mifepristone and their functions in primary human uterine leiomyoma
smooth muscle (LSM) cells and tissues from 39 premenopausal women.
Results: In primary LSM cells, progesterone significantly induced LAT2 mRNA levels, and this was
blocked by cotreatment with mifepristone. Progesterone did not alter 4F2hc mRNA levels, whereas
mifepristone significantly induced 4F2hc mRNA expression. Small interfering RNA knockdown of
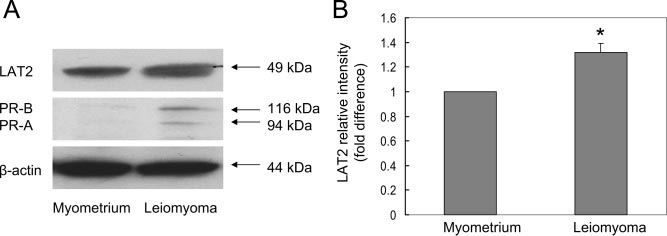
LAT2 or 4F2hc markedly increased LSM cell proliferation. LAT2, PR-B, and PR-A levels were signif-
icantly higher in freshly isolated LSM cells
vs. adjacent myometrial cells.
In vivo, mRNA levels of LAT2
and PR but not 4F2hc were significantly higher in leiomyoma tissues compared with matched
myometrial tissues.
Conclusion: We present evidence that progesterone and its antagonist mifepristone regulate the
amino acid transporter system LAT2/4F2hc in leiomyoma tissues and cells. Our findings suggest that
products of the LAT2/4F2hc genes may play important roles in leiomyoma cell proliferation. We
speculate that critical ratios of LAT2 to 4F2hc regulate leiomyoma growth.
(J Clin Endocrinol Metab
94: 4533– 4539, 2009)
Uterine leiomyomata (fibroids) are the most common leastonethirdofapproximately600,000hysterectomies
benign tumors in women, originating from uterine
performed annually in the United States (3).
smooth muscle cells. Symptomatic leiomyoma occurs in as
Although the etiology of uterine fibroids is unknown,
many as 30% of women over 35 yr of age (1). They are a
the growth of these tumors has been known to be depen-
frequent cause of abnormal uterine bleeding and are in-
dent on ovarian steroid hormones (4). Accumulating data
volved in recurrent pregnancy loss. Uterine leiomyomata
support the concept that progesterone plays an important
are the single most common underlying indication for hys-
role in regulating the growth of uterine leiomyoma (4, 5).
terectomy (2). Uterine leiomyomata are responsible for at
The role of progesterone and its receptor (PR) in the patho-
ISSN Print 0021-972X
ISSN Online 1945-7197
Abbreviations: Ct, Cycle threshold; 4F2hc, 4F2 heavy chain; GAPDH, glyceraldehyde-3-
Printed in U.S.A.
phosphate dehydrogenase; LAT2, L-type amino acid transporter 2; LSM, leiomyoma
Copyright 2009 by The Endocrine Society
smooth muscle; PCNA, proliferation cell nuclear antigen; PR, progesterone receptor;
doi: 10.1210/jc.2009-1286 Received June 18, 2009. Accepted August 26, 2009.
siRNA, small interfering RNA.
First Published Online October 6, 2009
J Clin Endocrinol Metab, November 2009, 94(11):4533– 4539
Luo
et al.
Progesterone Action, LAT2 and 4F2hc
J Clin Endocrinol Metab, November 2009, 94(11):4533– 4539
genesis of fibroids is highlighted by the following set of find-
leiomyoma, following a protocol approved by the Institutional
ings. First, PR has been observed to be higher in fibroid tissue
Review Board for Human Research of Northwestern University
compared with the myometrial tissue by several groups (6,
(Chicago, IL). The size of the tumors ranged from 3.5 to 15 cmin diameter. The subjects had not received any hormonal treat-
7). Secondly, the presence of PR in fibroid tissue was also
ment for at least three menstrual cycles before surgery. Each
shown to be positively correlated with their growth (8). Most
specimen was evaluated histologically by a pathologist. The cycle
importantly, progestins are able to reverse the reduction of
phase was estimated by the last menstrual period; this was con-
fibroid size that is induced by GnRH agonist therapy when
firmed by endometrial histology. Twenty-two samples were ob-
administered as add-back treatment (9, 10).
tained during the follicular phase, 12 during the luteal phase, and
The
in vivo effects of progesterone on leiomyoma growth,
five during menstruation. Of these 39 samples, 16 were used forcell culture experiments, whereas 29 were used for the tissue
however, are still not clearly understood. For example, the
experiments. We used tissues from six women to prepare cells
average leiomyoma size during the first trimester of preg-
and also for
in vivo studies. We isolated LSM cells from the
nancy is significantly higher compared with that before the
peripheral portions approximately 1 cm from the outer capsule
onset of pregnancy (11). On the other hand, leiomyoma size
of the leiomyoma, and cultured them as previously described
remains stable or can decrease slightly when the first and
with minor modifications (21). Immunocytochemistry using an
third trimesters of pregnancy are compared (12–14). Thus,
antibody against smooth muscle ␣-actin confirmed purity of the
progesterone may play dual roles regarding leiomyoma
cells (data not shown). Primary cells were used only up to thesecond passage to avoid changes in phenotype and gene expres-
growth under various
in vivo conditions.
sion. LSM cells were cultured in DMEM/F12 1:1 (GIBCO/BRL,
Previously, we and others have described functions of pro-
Grand Island, NY) containing 10% fetal bovine serum (Invitro-
gesterone-responsive genes that promote fibroid growth (5,
gen, Carlsbad, CA). The monolayer cultures at about 70% con-
15, 16). We recently performed a genome-wide chromatin
fluency were starved in serum-free medium overnight and treated
immunoprecipitation-cloning procedure to identify novel
with vehicle (ethyl alcohol 1:1000; Sigma-Aldrich, St. Louis,
binding sites of PR in chromatin isolated from leiomyoma
MO), progesterone (3 ⫻ 10⫺7 M; Sigma-Aldrich), or mifepris-tone (10⫺8-10⫺4 M; Sigma-Aldrich).
smooth muscle (LSM) cells. We noted that PR was recruited
All cell culture-based experiments were repeated using cells
to intron 12 of the L-type amino acid transporter 2 (LAT2)
from at least four subjects. One representative experiment was
gene (our unpublished observations). Here, we define novel
illustrated. Each cell culture-based experiment was carried out in
roles of LAT2 and its functional partner, a heavy chain of 4F2
triplicate replicates using cells in first or second passage.
antigen (4F2hc) in LSM cell fate.
LAT2 has a 12-membrane-spanning domain that medi-
RNA preparation and real-time quantitative PCR
ates Na⫹-independent amino acid exchange. It requires
Total RNA from LSM cells was extracted using Tri-reagent
an additional single-membrane-spanning domain pro-
(Sigma-Aldrich). cDNA was prepared with qScript cDNA Super-
tein, 4F2hc, to exert its function in the plasma membrane.
Mix (Quanta BioSciences, Inc., Gaithersburg, MD) from 2 g of
LAT2 and 4F2hc form a heterodimeric complex via a disul-
RNA. Primers against LAT2, 4F2hc, and the constitutively ex-
fide bond (17, 18). The mRNAs of LAT2 and 4F2hc are
pressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
expressed in most embryonic and adult tissues (18, 19).
were used as described in previous reports (22, 23). For LAT2, theforward and reverse primers were 5⬘-AATGCATTTGAGAATT-
LAT2 transports large neutral amino acids, as well as small
TCCAGGA-3⬘ and 5⬘-GAGCCCTGAAGGAAAGCCA-3⬘, re-
neutral amino acids (18, 19). LAT2 is involved mostly in the
spectively (22). For 4F2hc, the forward and reverse primers were
basolateral efflux step of transepithelial amino acid transport
5⬘-CTCAGGCAAGGCTCCTGACT-3⬘ and 5⬘-GGCAGGGT-
in the kidney and intestine (19). However, the exact local-
GAAGAGCATCA-3⬘, respectively (22). For GAPDH, the forward
ization of LAT2 on cell membranes is not fully known, and
and the reverse primers were 5⬘-GAAGGTGAAGGTCG-
the tissue distribution data are sometimes conflicting (20).
GAGTC-3⬘ and 5⬘-GAAGATGGTGATGGGATTTC-3⬘, respec-tively (23). Primer specificity was confirmed by the demonstration
The expression and functional properties of amino acid
of single peaks using dissociation curves after amplification of
transporters for supplying organic nutrition to cells have not
cDNA and a lack of amplification of genomic DNA.
been entirely clarified. Furthermore, nothing is known re-
Real-time quantitative PCR was performed to determine the
garding the expression and function of LAT2/4F2hc in hu-
relative amounts of each transcript using the DNA-binding dye
man uterine leiomyoma.
SYBR green (Applied Biosystems, Foster City, CA) and the ABIPrism 7900HT Detection System (Applied Biosystems). Cyclingconditions started at 50 C for 2 min, followed by 95 C for 10 min,then 40 cycles of 95 C for 15 sec and 60 C for 1 min. The cycle
Materials and Methods
threshold (C ) was placed at a set level where the exponential
increase in PCR amplification was approximately parallel be-
Tissue collection and primary cell culture
tween all samples. Relative fold change was calculated by com-
Human uterine leiomyoma and matched myometrial tis-
paring C values between the target gene and GAPDH as the
sues were obtained at surgery from 39 women (mean age, 40
reference guide. The 2⫺⌬⌬Ct method was used to analyze these
yr; range, 33– 48) undergoing hysterectomy for symptomatic
relative changes in gene expression (24).

J Clin Endocrinol Metab, November 2009, 94(11):4533– 4539
medium without antibiotics and incu-
bated for 24 h for mRNA and 72 h for
Western blot.
Cell viability assay
The effects of LAT2 siRNA or 4F2hc
siRNA on the viability of LSM cells was also
(fold change) 0.5
evaluated by the 3-(4, 5-dimethylthiazolyl-
2)-2, 5-diphenyltetrazolium bromide (MTT)
assay (Invitrogen). LSM cells were plated at
a density of 4.0 ⫻ 104 cells per well in 24-
well culture plate 1 d before transfection toachieve approximately 50% confluence at
the time of transfection. The transfection
was done as previously described and incu-
bated for 72 h. The MTT assay was per-
formed as described by the manufacturer'sprotocol with minor modifications. Briefly,
at 72 h from start of transfection, 40 l
MTT solution was added to each well, and
cells were incubated at 37 C for 4 h. Then
400 l of the sodium dodecyl sulfate-HCl
solution were added to each well and mixed
vehicle 10-8M 10-7M
10-6M 10-5M 10-4M
thoroughly. The plate was then incubated at
37 C for another 16 –18 h, and samples weretransferred to a 96-well plate and read at an
absorbance of 570 nm.
Protein isolation and
Cultured LSM and myometrial cells were
lysed using Mammalian Protein Extraction
Reagent (M-PER; Pierce, Rockford, IL) and
1 ⫻ protease inhibitor (Sigma-Aldrich). Pro-
tein concentrations were determined by col-
orimetric BCA protein Assay (Pierce), and
equal concentrations of total protein were
FIG. 1. Regulation of LAT2 and 4F2hc expression by progesterone or mifepristone. Primary
loaded in each well. Samples were subjected to
cultured LSM cells were starved in phenol red-free and serum-free DMEM/F12 (1:1) medium
PAGE (Bio-Rad, Hercules, CA) and trans-
overnight and then treated with vehicle (ethanol), progesterone, or mifepristone for different
ferred onto nitrocellulose membranes (In-
times. LAT2 (A, D, E) and 4F2hc (B, D, F) mRNA levels were measured by real-time PCR and
vitrogen). Membranes were probed using
normalized to GAPDH mRNA levels. LAT2 protein levels were evaluated by Western blot using
antibodies against -actin (Sigma-Aldrich),
a LAT2 peptide antibody (C). Blots were reprobed with a -actin antibody for loading control.
proliferation cell nuclear antigen (PCNA;
Results for A, B, D, E and F were reported as a fold change compared with cells treated with
Millipore, Burlington, MA), LAT2 (Santa
vehicle only and represented as the mean ⫾ SEM. All graphs were derived from one
Cruz Biotechnology, Santa Cruz, CA) or PR
representative experiment, and all experiments were repeated in triplicate in four subjects.
(PGR; courtesy of Dr. Dean Edwards, Baylor
*, P ⬍ 0.05, compared with vehicle treatment.
College of Medicine, Houston, TX). Anti-mouse and antigoat IgG conjugated to horse-
Small interfering RNA (siRNA)
radish peroxidase (Cell Signaling, Danvers,
RNA oligonucleotides directed against LAT2, 4F2hc, and a
MA) were used as secondary antibodies. Immunoreactive bands
mismatch negative control siRNA were purchased from Dhar-
were visualized using the ECL-detection system (GE Healthcare,
macon (Lafayette, CO). LSM cells were plated at a density of
Piscataway, NJ). Quantification of chemiluminescence signal in-
1.0 ⫻ 106 cells per 10-cm dish in phenol free DMEM/F12 con-
tensity was performed after completion of all autoradiographic
taining 10% charcoal stripped fetal bovine serum, but lacking
studies with ImageJ software (National Institutes of Health, Be-
gentamycin/amphotericin B, 1 d before transfection to achieve
thesda, MD).
approximately 50% confluence at the time of transfection. Onthe day of transfection, the RNAiMAX lipofectamine-based re-agent was combined in conjunction with 200 nM siRNA du-
plexes diluted in Opti-Mem I (Invitrogen) and applied to the cells
Statistical significance was determined by Student's t test and
according to the manufacturer's instructions. Five hours after the
one-way ANOVA followed by Fisher's protected least signifi-
start of the transfection, the medium was changed to growth
cance difference test. Significance was accepted at P ⬍ 0.05.


Luo et al.
Progesterone Action, LAT2 and 4F2hc
J Clin Endocrinol Metab, November 2009, 94(11):4533– 4539
but it did not influence LAT2 mRNA
level at any concentration (Fig. 1D).
To elucidate whether mifepristone
could block the action of progesterone,
we treated LSM cells with progester-
one alone, mifepristone alone, or their
(fold change) 0.2
combination for 4 h. Consistently,
mifepristone alone did not affect LAT2
expression, but it abolished progester-
one-induced LAT2 mRNA level (Fig.
1E). On the other hand, progesterone
did not influence 4F2hc mRNA levels,
whereas mifepristone significantly in-
duced them (Fig. 1F).
Relative intensity
Knockdown of LAT2 is associated
with increased LSM cell
FIG. 2. The effects of knocking down LAT2 on LSM cell proliferation. LSM cells were
We performed knockdown of en-
transfected with control siRNA or LAT2 siRNA for 72 h. Knockdown efficiency and specificity
dogenous LAT2 to investigate how it
of the LAT2 gene were examined by both real-time PCR (A) and Western blot (B). Cell
may be involved in the regulation of
proliferation was analyzed by measuring PCNA protein level with mouse antihuman PCNA
LSM cell proliferation by examining
antibody (B). Blots were reprobed with a -actin antibody for loading control. Western blotdensities were quantified with ImageJ software (C). The effect of knocking down LAT2 on
PCNA protein levels and MTT assays.
LSM cell proliferation was confirmed with MTT assay (D). Data for A (represented as mean ⫾
Real-time PCR demonstrated that
SEM) and B were from one representative experiment and were repeated in four subjects. Data
siRNA against LAT2 significantly re-
for C and D show the mean ⫾ SEM from four subjects. Each experiment was done in triplicate.
*, P ⬍ 0.05, compared with control siRNA.
duced its endogenous mean mRNAlevel by over 90% (Fig. 2A). Westernblot analysis confirmed that LAT2 pro-
tein level was also markedly decreased by LAT2 siRNA(Fig. 2, B and C). Knockdown of LAT2 increased the pro-
Regulation of LAT2 and 4F2hc mRNA levels by
liferation marker PCNA (Fig. 2, B and C), suggesting that
progesterone or mifepristone in LSM cells
LAT2 inhibited proliferation of LSM cells. The effect of
Our preliminary dose-response (10⫺10-10⫺6 M) exper-
siRNA LAT2 knockdown on the proliferation of LSM
iment showed that progesterone regulated LAT2 expres-
cells was confirmed by MTT assay, which reflects the total
sion in LSM cells at 10⫺8, 10⫺7, and 10⫺6 M concentra-
number of viable cells. As shown in Fig. 2D, knockdown
tions; peak regulation occurred at 10⫺6 M dose after 4 h of
of LAT2 significantly increased the number of viable cells.
treatment (data not shown). We chose to use the 3 ⫻ 10⫺7 M
Knockdown of LAT2 also increased the proliferation of
concentration of progesterone to further examine its ef-
myometrial cells from matched tissues (data not shown).
fects on LAT2 and 4F2hc gene expression.
Real-time quantitative PCR analyses were performed
Knockdown of 4F2hc is also involved in increased
after LSM cells were incubated with progesterone (3 ⫻
LSM cell proliferation
10⫺7 M) for 2 and 4 h. LAT2 mRNA levels were signifi-
We performed knockdown of endogenous 4F2hc to
cantly induced by progesterone at both 2 and 4 h (Fig. 1A).
investigate its effects on proliferation of LSM cells. Real-
However, progesterone did not affect 4F2hc mRNA lev-
time PCR analysis showed that 4F2hc siRNA reduced en-
els (Fig. 1B). Treatment at the same concentration for
dogenous 4F2hc mRNA expression by nearly 80% (Fig.
72 h also significantly up-regulated LAT2 protein levels
3A). 4F2hc protein expression could not be demonstrated
because a commercial antibody was not available. 4F2hc
To determine whether progesterone antagonist mife-
knockdown by siRNA significantly increased PCNA level
pristone affects LAT2 or 4F2hc expression differentially,
(Fig. 3, B and C). Alterations in LSM cell proliferation
we treated LSM cells with various concentrations (10⫺8-
were also verified by the MTT assay. Figure 3D illustrates
10⫺4 M) of mifepristone for 4 h. In contrast to the regu-
that 4F2hc knockdown by siRNA modestly but signifi-
lation of these two genes by progesterone, mifepristone
cantly increased the number of viable cells. These results
induced 4F2hc mRNA levels in a dose-dependent manner,
suggest that 4F2hc may also be involved in inhibition of


J Clin Endocrinol Metab, November 2009, 94(11):4533– 4539
4F2hc, and PR were normalized to
GAPDH, and then the mRNA levels in
leiomyoma tissues were expressed as a
multiple of that value in matched myo-
metrial tissues, allowing the detection
of relative mRNA differences between
(fold change) 0.2
the two tissues.
As shown in Fig. 5, mean LAT2 and
PR mRNA levels were significantly
higher in leiomyoma than myometrial
tissues. However, there was no signifi-
cant difference in mRNA levels of 4F2hc
between leiomyoma and matched myo-
(fold change) 0.6
metrial tissues.
PCNA relative intensity
FIG. 3. The effects of knocking down 4F2hc on LSM cell proliferation. LSM cells were
In this study, we have demonstrated
transfected with control siRNA or 4F2hc siRNA for 72 h. The cells were then harvested for
that progesterone and its antagonist
analysis. Knockdown efficiency and specificity of the 4F2hc gene were examined by real-timePCR (A). LSM cells were harvested for determination of cell proliferation by measuring of
mifepristone regulated LAT2 and 4F2hc
PCNA protein level with mouse antihuman PCNA antibody (B). Western blot densities were
expression. LAT2 knockdown studies
quantified with ImageJ software (C). The effect of knockdown 4F2hc on LSM cell proliferation
suggested that LAT2 inhibits LSM cell
was confirmed with MTT assay (D). Blots were reprobed with a -actin antibody, which wasused as a control. 4F2hc mRNA measured by real-time PCR was normalized to GAPDH mRNA.
growth, although its product is consid-
Data for A (represented as mean ⫾ SEM) and B were from one representative experiment and
ered to be an amino acid transporter.
were repeated in four subjects. Data for C and D are shown as the mean ⫾ SEM from four
Thus, the role of LAT2 in leiomyoma
subjects. Each experiment was done in triplicate. *, P ⬍ 0.05, compared with control siRNA.
pathology may seem paradoxical atfirst sight. The functional properties of
LSM cell growth. Similar to its effects on LSM cell pro-
LAT2 for supplying organic nutrition to cells have not
liferation, knockdown of 4F2hc stimulated myometrial
been entirely clarified. It has been reported that LAT2
cell proliferation (data not shown).
operates as an amino acid exchanger (19, 25). There is alsoevidence to suggest that amino acid efflux via LAT2 is not
Expression of LAT2 and PR in cultured LSM cells
dependent upon extracellular amino acids (18, 26). The
and matched myometrial cells
net direction of the transport of large neutral amino acids
The effects of progesterone on target tissues are medi-
by LAT2 is believed to depend on the unidirectional trans-
ated by PR. Previous studies have demonstrated that PR
porters that are coexpressed in the cells. Thus, the role of
levels are higher in LSM cells than in myometrial cells (6).
LAT2 is most likely an equilibration of the amino acid
To determine whether cell-specific differences in LAT2
distribution across the two membranes, whereas other
levels are accompanied by comparable differences in PR,
transporters determine the actual net amino acid flux (27).
LAT2 and PR protein expression in LSM cells and
Knockdown of LAT2 in LSM cells might have changed the
matched myometrial cells was examined using Western
equilibration of the amino acid distribution across the cell
blot analysis. Compared with myometrial cells, both PR
membrane, which could then alter the net flux of amino
and LAT2 protein expression was higher in cultured LSMcells (Fig. 4, A and B).
acid determined by other transporters. The final outcome,therefore, may be the increase of cell growth.
Gene expression of LAT2, 4F2hc, and PR in
We also found that knockdown of 4F2hc increased the
leiomyoma and matched myometrial tissues
proliferation of LSM cells, suggesting that this gene may
To understand the in vivo relevance of progesterone-
inhibit leiomyoma growth. Our result is different from
regulated LAT2 and 4F2hc expression in leiomyoma, we
what was demonstrated in a previous study (28), which
analyzed mRNA levels of PR, LAT2, and 4F2hc genes in
showed that a monoclonal antibody against 4F2hc anti-
human leiomyoma and matched myometrial tissues from
gen inhibited lymphocyte activation and cell proliferation.
29 subjects. To allow comparisons of data obtained from
This difference may be due to the different cell systems and
samples of different subjects, the mRNA levels of LAT2,
methods used. To our knowledge, the present study is the
Luo et al.
Progesterone Action, LAT2 and 4F2hc
J Clin Endocrinol Metab, November 2009, 94(11):4533– 4539
to 4F2hc ratio in leiomyoma tissue mayfavor its growth. Further studies areneeded to clearly understand this com-plex mechanism.
We find that progesterone or mife-
pristone regulates LAT2 or 4F2hc ex-pression via PR. PR exists as two iso-forms, PR-A and PR-B (30). In humans,PR-B and PR-A may have distinct or
FIG. 4. Comparison of LAT2 and PR protein levels in cultured LSM cells with matched
similar functions depending on the pro-
myometrial cells. Cell lysates were prepared for immunoblotting with anti-LAT2, anti-PR and
moter context and cell type (31, 32).
-actin (loading control) antibodies (A) and quantified with ImageJ software (B). Data in A
Thus, it seems justified to further
from one representative result were reproduced in cells from four patients. Data in B
evaluate the effects of each isoform on
represent the mean ⫾ SEM from four subjects. *, P ⬍ 0.05, compared with myometrial cells.
LAT2 or 4F2hc expression in LSM
first to demonstrate the function of LAT2/4F2hc through
knockdown of these genes, and the first to report the ex-
Information on the hormonal mechanisms regulating
pression and function of LAT2/4F2hc in leiomyoma.
system-L transporter activity is still scarce (33–35). Ste-
It should be noted that LAT2 requires 4F2hc to func-
roid hormones may act via multiple mechanisms. They
tion normally in the plasma membrane (17, 18). Knocking
affect not only the transcriptional modulation of target
down 4F2hc could lead to a loss of function of LAT2.
genes via intracellular receptors within minutes to hours
Thus, our observation that knockdown of 4F2hc stimu-
but also elicit rapid nongenomic effects within seconds to
lated leiomyoma cell proliferation is consistent with the
minutes. Steroid hormones have been shown to enhance or
effect of LAT2 knockdown on the same cell type. In vivo,
diminish amino acid transport depending on the nature of
however, LAT2 but not 4F2hc expression in leiomyoma
the hormone. The stimulation may involve either in-
tissue was higher. It is likely that critical ratios of LAT2 to
creased carrier synthesis or rapid nongenomic effects at
4F2hc determine the net in vivo effects of this L-type
the cell membrane (36, 37), whereas inhibition may in-
amino acid transporter system in leiomyoma tissue. To
volve synthesis of a labile protein that either decreases the
add a further twist, treatment with progesterone increases,
rate of synthesis or increases the rate of degradation of a
whereas mifepristone decreases, the LAT2 to 4F2hc ratio.
component of the transport system (38).
Evidence from a number of laboratories suggests that pro-
In summary, we demonstrated the functions of two
gesterone stimulates, whereas mifepristone inhibits
genes involved in amino acid transport through the cell
leiomyoma growth (4, 5, 29). The sum of these observa-
membrane in leiomyoma cell growth. The products of
tions is consistent with a model that a higher in vivo LAT2
these genes heterodimerize to regulate amino acid trans-port and also regulate leiomyoma proliferation; more-over, progesterone or an antiprogestin regulates their ex-
pression. We speculate that critical ratios of LAT2 to
4F2hc may regulate leiomyoma growth.
We thank Dr. Dean Edwards (Baylor College of Medicine, Hous-ton, TX) for the PR antibody.
(fold difference)
Tissue mRNA levels
Address all correspondence and requests for reprints to: Serdar
E. Bulun, Division of Reproductive Biology Research, FeinbergSchool of Medicine, Northwestern University, 303 East Superior
Street, Suite 4-123, Chicago, Illinois 60611. E-mail: s-bulun@
FIG. 5. LAT2, 4F2hc, and PR mRNA levels in human leiomyoma and
This work was supported by National Institutes of Health
matched myometrial tissues. Overall, 58 samples from 29 patients
Grant HD46260.
were analyzed; 29 samples were obtained from leiomyoma and 29
Disclosure Summary: X.L., P.Y., S.R., H.I., Z.L., M.E.P.,
from adjacent myometrial tissues. To allow comparisons of data
H.Z., and E.E.M. have nothing to declare. S.E.B. serves as a
obtained from samples from different patients, mRNA levels in themyometrial tissues were normalized to 1. The data were shown as the
consultant for the pharmaceutical companies Repros, Medit-
mean ⫾ SEM. *, P ⬍ 0.01, compared with myometrial tissues.
rina, and Novartis.
J Clin Endocrinol Metab, November 2009, 94(11):4533– 4539
20. del Amo EM, Urtti A, Yliperttula M 2008 Pharmacokinetic role of
L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci
1. Vollenhoven BJ, Lawrence AS, Healy DL 1990 Uterine fibroids: a
clinical review. Br J Obstet Gynaecol 97:285–298
21. Rossi MJ, Chegini N, Masterson BJ 1992 Presence of epidermal
2. Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB
growth factor, platelet-derived growth factor, and their receptors in
1994 Hysterectomy in the United States, 1988 –1990. Obstet Gy-
human myometrial tissue and smooth muscle cells: their action in
necol 83:549 –555
smooth muscle cells in vitro. Endocrinology 130:1716 –1727
3. Mo¨ller C, Hoffmann J, Kirkland TA, Schwede W 2008 Investiga-
22. Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A,
tional developments for the treatment of progesterone-dependent
Babu E, Tachampa K, Anzai N, Iribe Y, Endou H 2002 Character-
diseases. Expert Opin Investig Drugs 17:469 – 479
ization of the system L amino acid transporter in T24 human bladder
4. Marsh EE, Bulun SE 2006 Steroid hormones and leiomyomas. Ob-
carcinoma cells. Biochim Biophys Acta 1565:112–121
stet Gynecol Clin North Am 33:59 – 67
23. Ishikawa H, Reierstad S, Demura M, Rademaker AW, Kasai T,
5. Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H,
Inoue M, Usui H, Shozu M, Bulun SE 2009 High aromatase ex-
Xue Q, Reierstad S, Innes J, Thung S, Kim JJ, Xu E, Bulun SE 2007
pression in uterine leiomyoma tissues of African-American women.
Progesterone receptor regulates Bcl-2 gene expression through direct
J Clin Endocrinol Metab 94:1752–1756
binding to its promoter region in uterine leiomyoma cells. J Clin
24. Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression
Endocrinol Metab 92:4459 – 4466
data using real-time quantitative PCR and the 2(-⌬⌬C(T)) method.
6. Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington
Methods 25:402– 408
MS, Erickson TE, Warner C, Keenan EJ, Clinton GM 1993 Proges-
25. Pineda M, Ferna´ndez E, Torrents D, Este´vez R, Lo´pez C, Camps M,
terone receptor messenger ribonucleic acid and protein are overex-
Lloberas J, Zorzano A, Palacín M 1999 Identification of a mem-
pressed in human uterine leiomyomas. Am J Obstet Gynecol 169:
brane protein, LAT-2, that co-expresses with 4F2 heavy chain, an
L-type amino acid transport activity with broad specificity for small
7. Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjo¨blom P, Norgren
and large zwitterionic amino acids. J Biol Chem 274:19738 –19744
A, Lindblom B 1998 Sex steroid receptors in human myometrium and
26. Liu X, Charrier L, Gewirtz A, Sitaraman S, Merlin D 2003 CD98
fibroids: changes during the menstrual cycle and gonadotropin-releas-ing hormone treatment. J Clin Endocrinol Metab 83:4092–4096
and intracellular adhesion molecule I regulate the activity of amino
8. Ichimura T, Kawamura N, Ito F, Shibata S, Minakuchi K, Tsujimura
acid transporter LAT-2 in polarized intestinal epithelia. J Biol Chem
A, Umesaki N, Ogita S 1998 Correlation between the growth of
uterine leiomyomata and estrogen and progesterone receptor con-
27. Meier C, Ristic Z, Klauser S, Verrey F 2002 Activation of system L
tent in needle biopsy specimens. Fertil Steril 70:967–971
heterodimeric amino acid exchangers by intracellular substrates.
9. Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA,
EMBO J 21:580 –589
Byrd W, Roark M, Steinkampf MP 1993 An evaluation of the effect of
28. Yagita H, Masuko T, Hashimoto Y 1986 Inhibition of tumor cell
gonadotropin-releasing hormone analogs and medroxyprogesterone
growth in vitro by murine monoclonal antibodies that recognize a
acetate on uterine leiomyomata volume by magnetic resonance imag-
proliferation-associated cell surface antigen system in rats and hu-
ing: a prospective, randomized, double blind, placebo-controlled,
mans. Cancer Res 46:1478 –1484
crossover trial. J Clin Endocrinol Metab 76:1217–1223
29. Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS 1993
10. Friedman AJ, Barbieri RL, Doubilet PM, Fine C, Schiff I 1988 A ran-
Regression of uterine leiomyomata in response to the antiprogest-
domized, double-blind trial of a gonadotropin releasing-hormone ag-
erone RU 486. J Clin Endocrinol Metab 76:513–517
onist (leuprolide) with or without medroxyprogesterone acetate in the
30. Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H,
treatment of leiomyomata uteri. Fertil Steril 49:404–409
Chambon P 1990 Two distinct estrogen-regulated promoters gen-
11. Rosati P, Exacousto s C, Mancuso S 1992 Longitudinal evaluation
erate transcripts encoding the two functionally different human pro-
of uterine myoma growth during pregnancy. A sonographic study.
gesterone receptor forms A and B. EMBO J 9:1603–1614
J Ultrasound Med 11:511–515
31. Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW,
12. Aharoni A, Reiter A, Golan D, Paltiely Y, Sharf M 1988 Patterns of
McDonnell DP 1993 Human progesterone receptor A form is a cell-
growth of uterine leiomyomas during pregnancy. A prospective lon-
and promoter-specific repressor of human progesterone receptor B
gitudinal study. Br J Obstet Gynaecol 95:510 –513
function. Mol Endocrinol 7:1244 –1255
13. Hammoud AO, Asaad R, Berman J, Treadwell MC, Blackwell S,
32. Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP 1994 The
Diamond MP 2006 Volume change of uterine myomas during preg-
A and B isoforms of the human progesterone receptor operate
nancy: do myomas really grow? J Minim Invasive Gynecol 13:386–390
through distinct signaling pathways within target cells. Mol Cell Biol
14. Neiger R, Sonek JD, Croom CS, Ventolini G 2006 Pregnancy-related
changes in the size of uterine leiomyomas. J Reprod Med 51:671–674
33. Laverty G, Elbrønd VS, Arnason SS, Skadhauge E 2006 Endocrine
15. Maruo T, Matsuo H, Shimomura Y, Kurachi O, Gao Z, Nakago S,
regulation of ion transport in the avian lower intestine. Gen Comp
Yamada T, Chen W, Wang J 2003 Effects of progesterone on growth
Endocrinol 147:70 –77
factor expression in human uterine leiomyoma. Steroids 68:817–824
34. Mate A, Barfull A, Hermosa AM, Go´mez-Amores L, Va´zquez CM,
16. Maruo T, Ohara N, Wang J, Matsuo H 2004 Sex steroidal regula-
tion of uterine leiomyoma growth and apoptosis. Hum Reprod Up-
Planas JM 2006 Regulation of sodium-glucose cotransporter
date 10:207–220
SGLT1 in the intestine of hypertensive rats. Am J Physiol Regul
17. Kim DK, Kim IJ, Hwang S, Kook JH, Lee MC, Shin BA, Bae CS,
Integr Comp Physiol 291:R760 –R767
Yoon JH, Ahn SG, Kim SA, Kanai Y, Endou H, Kim JK 2004 System
35. Moreto´ M, Cristia E, Pe´rez-Bosque A, Afzal-Ahmed I, Amat C,
L-amino acid transporters are differently expressed in rat astrocyte
Naftalin RJ 2005 Aldosterone reduces crypt colon permeability dur-
and C6 glioma cells. Neurosci Res 50:437– 446
ing low-sodium adaptation. J Membr Biol 206:43–51
18. Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai
36. Boldyreff B, Wehling M 2004 Aldosterone: refreshing a slow hor-
Y 1999 Identification and functional characterization of a Na⫹-
mone by swift action. News Physiol Sci 19:97–100
independent neutral amino acid transporter with broad substrate
37. Boldyreff B, Wehling M 2003 Rapid aldosterone actions: from the
selectivity. J Biol Chem 274:19745–19751
membrane to signaling cascades to gene transcription and physio-
19. Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Ku¨hn LC
logical effects. J Steroid Biochem Mol Biol 85:375–381
1999 LAT2, a new basolateral 4F2hc/CD98-associated amino acid
38. Lerner J 1985 Effectors of amino acid transport processes in animal cell
transporter of kidney and intestine. J Biol Chem 274:34948–34954
membranes. Comp Biochem Physiol A Comp Physiol 81:713–739
Source: http://itp.w3.kanazawa-u.ac.jp/achievements/pdf/002.pdf
Calcium hydroxylapatite associated softtissue necrosis: A case report and treatmentguideline Lauren Tracy , James Ridgway , J. Stuart Nelson Nelson Lowe , Brian Wong a Department of Otolaryngology e Head and Neck Surgery, University of California Irvine, 101 The CityDrive, Bldg. 56, Suite 500, Orange, CA 92868, USAb Larrabee Center for Plastic Surgery, 600 Broadway, Suite 280, Seattle, WA 98122, USAc Beckman Laser Institute and Medical Clinic, University of California Irvine, 1002 Health Sciences RoadEast, Irvine, CA 92612, USAd 999 North Tustin Avenue, Suite 117, Santa Ana, CA 92705, USA
that their reproductive organs can be One of the basic tenets of the animal exploited, many believe that the original liberation movement is that there is no point of patriarchy was to control the moral difference between human and reproductive systems of women. non-human animals. If something ought not be done to humans, then it ought not be done to animals. And vice-versa.












