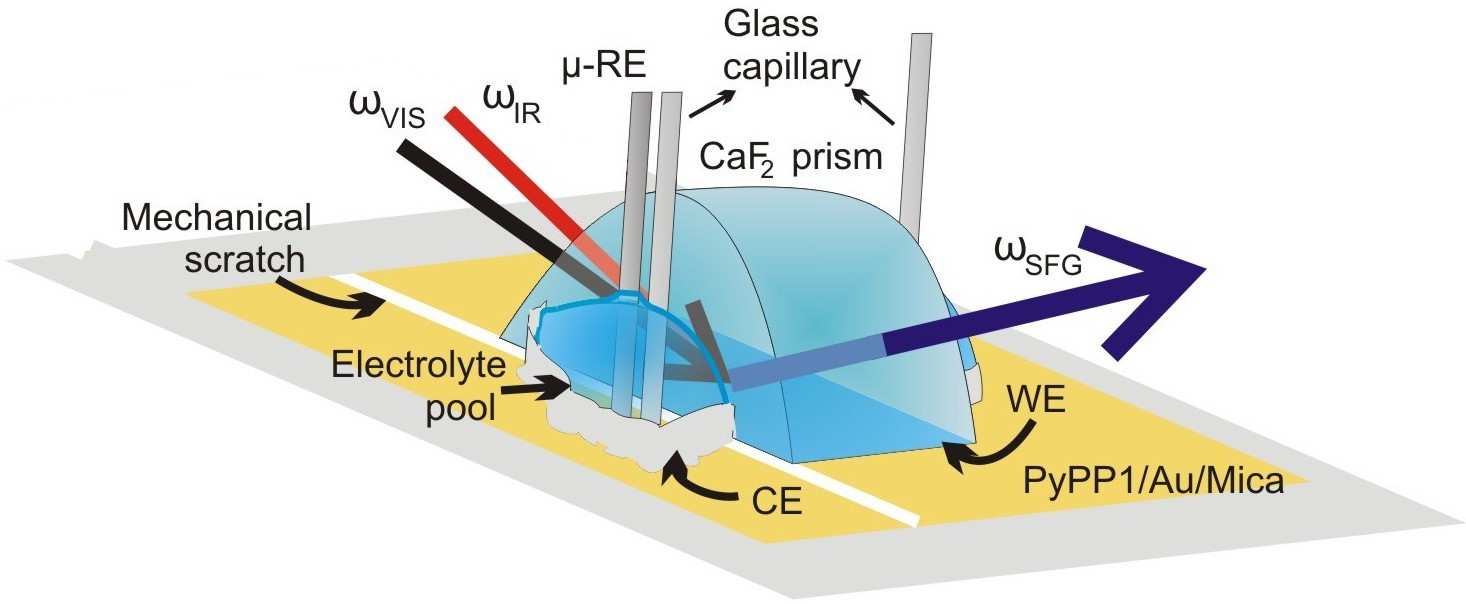
Microsoft word - 03reisepraktisches09.doc
Getränke Havanna vo „Staubfänger"-Phalanx fürs heimatliche Wohnzimmer Havanna von A bis Z Adressen Nummer 169 A an der Ecke der Straßen Virtudes und Amistad. Die Adressen in Havanna sind recht Damit man sich leichter nach dem Weg verwirrend – auf den ersten Blick. Bei erkundigen kann, sind in diesem City- näherer Betrachtung sind sie allerdings