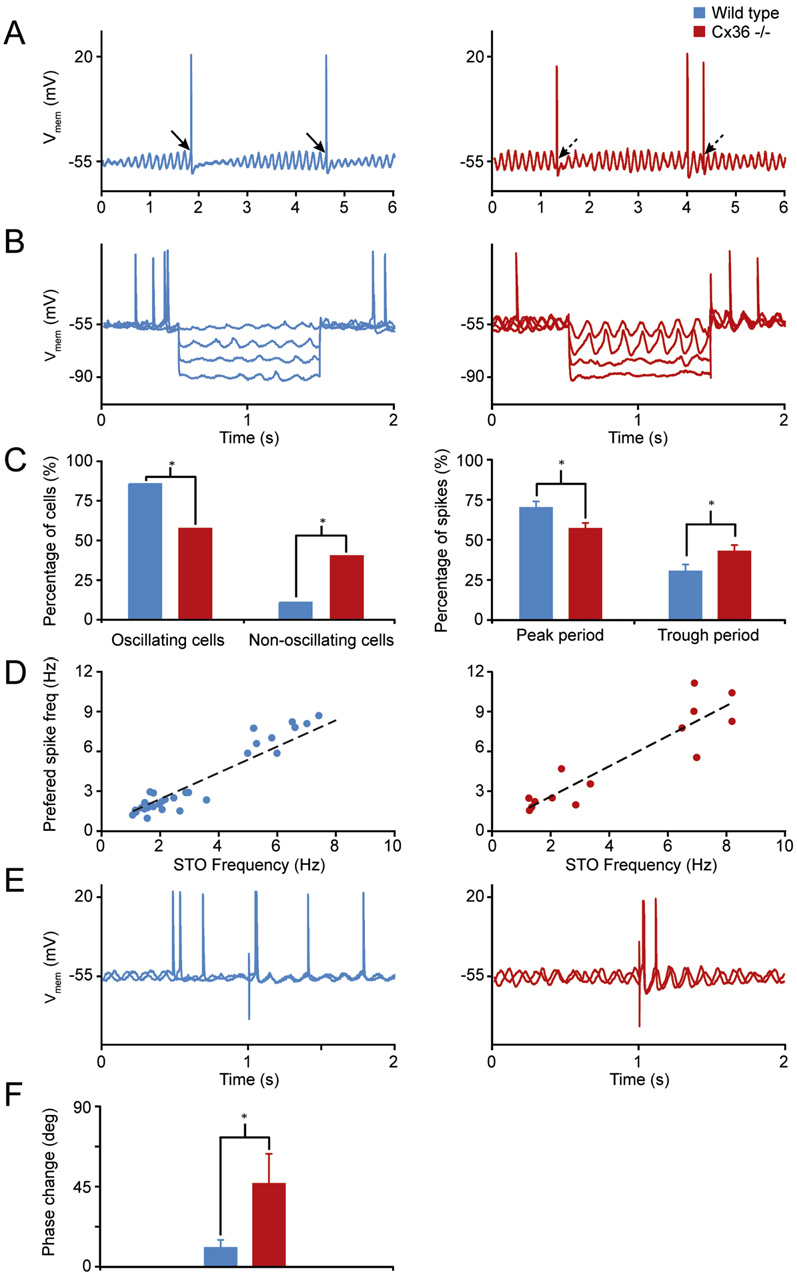
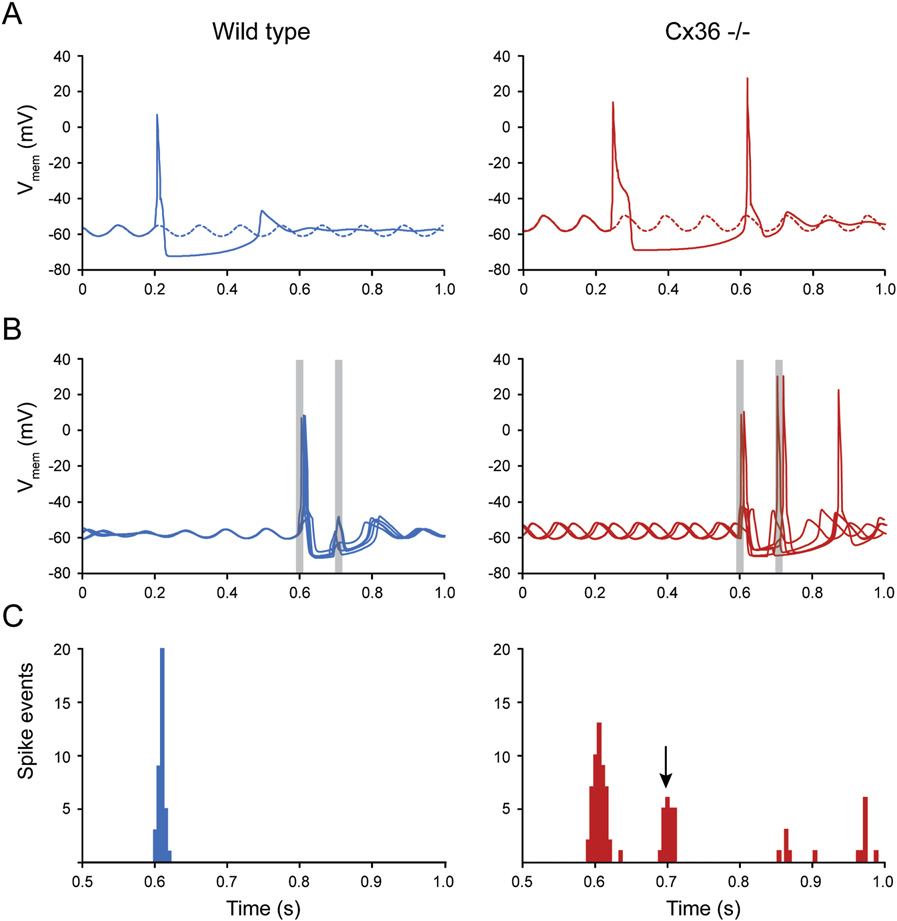
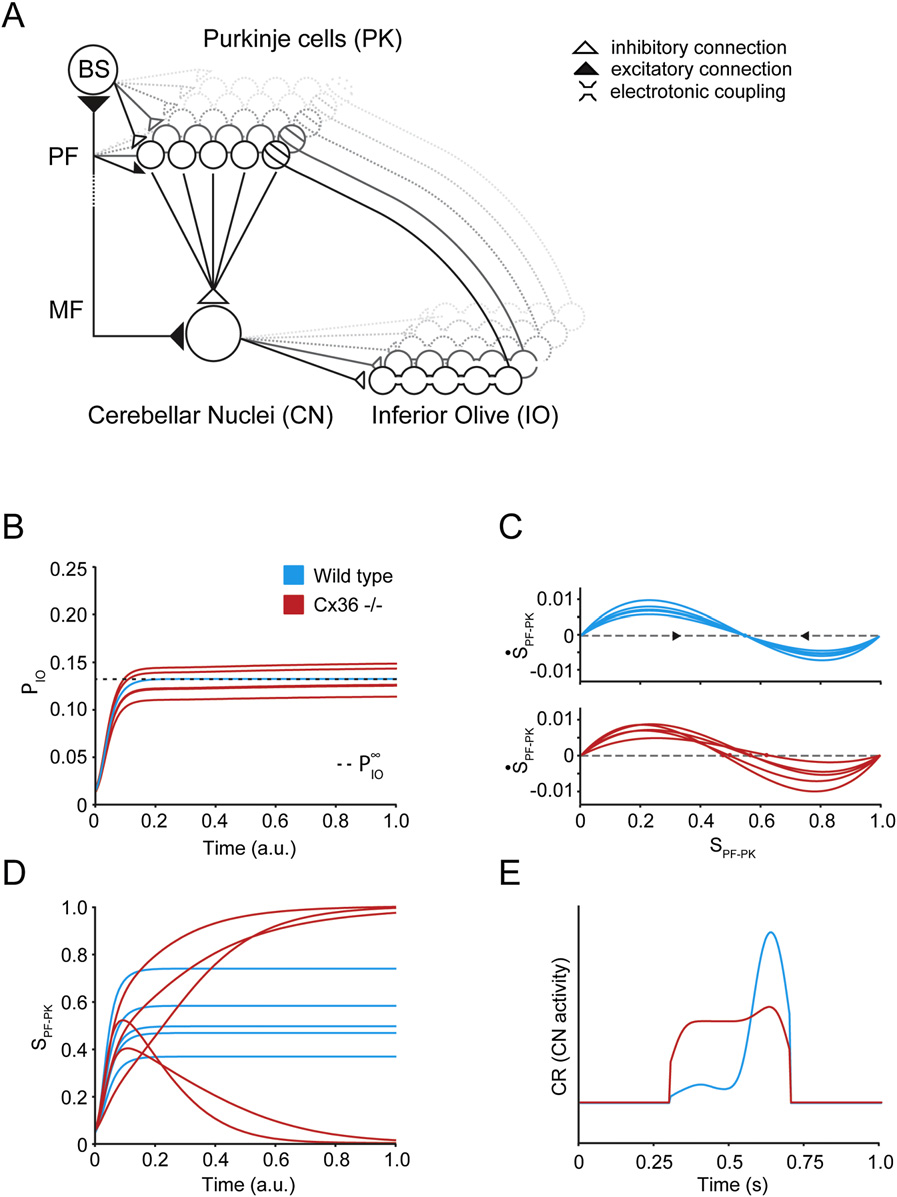
Q90texte.doc
Table des matières PATHOLOGIE RHINO-SINUSIENNE AIGUË .2 Rhinites aiguës.2 Sinusites aiguës .2 L'ethmoïdite aiguë de l'enfant .5 PATHOLOGIE RHINO-SINUSIENNE CHRONIQUE.7 Classification des pathologies rhinosinusiennes chroniques .7 Diagnostic de rhinosinusites chronique .7 Les sinusites antérieures de la face.7 Les pan-sinusites bilatérales et symétriques .8