
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Healthislife.org
The Journal of Neuroscience, August 1, 2002,
22(15):6321–6324
Caffeine Induces Dopamine and Glutamate Release in the Shell of
the Nucleus Accumbens
Marcello Solinas,1
Sergi Ferre´,1
Zhi-Bing You,2
Marzena Karcz-Kubicha,1
Patrizia Popoli,3
and
Steven R. Goldberg1
Sections of 1
Preclinical Pharmacology and 2
Behavioral Neuroscience, Behavioral Neuroscience Branch, National Instituteon Drug Abuse, National Institutes of Health Intramural Research Program, Baltimore, Maryland 21224, and3
Department of Pharmacology, Istituto Superiore di Sanita, 00161 Rome, Italy
An increase in the extracellular concentration of dopamine in
adenosine A1 receptor antagonist but not by a selective aden-
the nucleus accumbens (NAc) is believed to be one of the main
osine A2A receptor antagonist. This suggests that caffeine,
mechanisms involved in the rewarding and motor-activating
because of its ability to block adenosine A1 receptors, shares
properties of psychostimulants such as amphetamines and
neurochemical properties with other psychostimulants, which
cocaine. Using
in vivo microdialysis in freely moving rats, we
could contribute to the widespread consumption of caffeine-
demonstrate that systemic administration of behaviorally rele-
vant doses of caffeine can preferentially increase extracellularlevels of dopamine and glutamate in the shell of the NAc. These
Key words: caffeine; adenosine; dopamine; glutamate; ac-
effects could be reproduced by the administration of a selective
Caffeine is the most consumed psychoactive drug in the world.
for a dopamine-releasing effect of behaviorally relevant doses of
Although it is a psychostimulant, it is not generally considered a
caffeine in brain regions that may mediate its psychostimulant
typical drug of dependence (Daly and Fredholm, 1998). Other
actions has not yet been obtained. Only a previous study by
psychostimulants, such as amphetamines and cocaine, elevate the
Morgan and Vestal (1989), using
in vivo voltammetry, indicated
extracellular concentration of dopamine in the nucleus accum-
that low doses of caffeine increase dopamine release in the rat
bens (NAc); this is believed to be one of the main mechanisms
caudate putamen. In the present study, by using
in vivo microdi-
involved in the rewarding and motor-activating properties of
alysis in freely moving rats, we demonstrate that systemic admin-
these drugs (Pontieri et al., 1995; Wise and Bozarth, 1987). In
istration of behaviorally relevant doses of caffeine can preferen-
addition, augmented extracellular levels of glutamate in the NAc
tially increase the extracellular levels of dopamine and glutamate
may be involved in the central effects of psychostimulants (Reid et
in the shell of the NAc.
al., 1997). The main mechanism of action of caffeine in the brain
seems to be a nonselective competitive blockade of adenosine
MATERIALS AND METHODS
receptors, in particular adenosine A1 receptors and A2A recep-
Subjects and drugs. Male Sprague Dawley rats, weighing 300 –350 gm,
tors (Daly and Fredholm, 1998). In the striatum, adenosine plays
were used in all experiments. Animals were maintained in facilities fully
an important role as a modulator of both dopamine and gluta-
accredited by the American Association for the Accreditation of Labo-
mate neurotransmission. At a presynaptic level, adenosine,
ratory Animal Care; all experimentation was conducted in accordance
with the guidelines of the Institutional Care and Use Committee of the
mostly by acting on adenosine A1 receptors localized in nerve
Intramural Research Program, National Institute on Drug Abuse
terminals, inhibits dopamine and glutamate release (Wood et al.,
(NIDA), National Institutes of Health, the directives of the
Principles of
1989; Okada et al., 1996; Flagmeyer et al., 1997; Golembiowska
Laboratory Animal Care (National Institutes of Health publication num-
and Zylewska, 1997). At a postsynaptic level, adenosine decreases
ber 85-23, revised 1985), and the Council of the European Communities
(86/809/EEC). Caffeine, the adenosine A1 antagonist 8-cyclopentyl-
dopaminergic neurotransmission by means of specific antagonis-
theophylline (CPT), and the adenosine A2A antagonist 5-amino-7-(2-
tic interactions between adenosine and dopamine receptors
(Ferre´ et al., 1997). Thus, caffeine, by antagonizing the effects of
(SCH 58261) were administered intraperitoneally in all experiments.
endogenous adenosine, can facilitate dopaminergic neurotrans-
Caffeine was dissolved in warm saline, CPT was dissolved in saline with
mission by stimulating dopamine release and by potentiating the
a minimal amount of 1N NaOH, and the A2A receptor antagonist SCH
58261 was dissolved in dimethylsulfoxide.
effects of dopamine receptor stimulation (Ferre´ et al., 1997).
Motor activity experiments. Motor activity was measured in automated
Although the latter mechanism is very well established, evidence
activity meters (Automex II; Columbus Instruments, Columbus, OH) in
habituated animals (30 min), as described in detail previously (Popoli et
al., 1998). Total horizontal motor activity (total accumulated counts of a
Received Feb. 11, 2002; revised May 2, 2002; accepted May 10, 2002.
single photocell interruption) was collected for 60 min after intraperito-
This work was supported by the Intramural Research Program of the National
neal administration of caffeine (3, 10, 30, and 100 mg/kg) or saline. A
Institute on Drug Abuse, National Institutes of Health.
Correspondence should be addressed to Dr. Steven R. Goldberg, National Insti-
one-way ANOVA followed by a Dunnett's
post hoc test was used for
tute on Drug Abuse, National Institutes of Health Intramural Research Program,
Behavioral Neuroscience Branch, Preclinical Pharmacology Section, 5500 Nathan
In vivo
microdialysis experiments. Concentric microdialysis probes
Shock Drive, Baltimore, MD 21224. E-mail:
[email protected].
were prepared as described previously (Pontieri et al., 1995). Animals
Copyright 2002 Society for Neuroscience 0270-6474/02/226321-04$15.00/0
were anesthetized with Equithesin (NIDA Pharmacy, Baltimore, MD),
6322 J. Neurosci., August 1, 2002, 22(15):6321–6324
Solinas et al. • Caffeine-Induced Dopamine and Glutamate Release
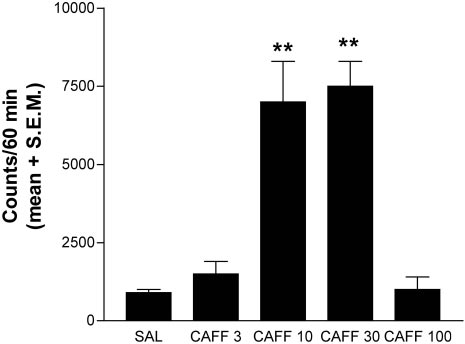
Figure 1. Total horizontal motor activity after intraperitoneal adminis-
tration of caffeine in habituated rats. The results represent means ⫾ SEM
of the accumulated motor activity counts during the first 60 min period of
observation (n ⫽ 6 per group). Significant motor activation was obtained
with caffeine in a dose of 10 mg/kg (CAFF 10) and caffeine in a dose of
30 mg/kg (CAFF 30). **p ⬍ 0.01 compared with the group treated with
saline (SAL).CAFF 3 and CAFF 100 indicate 3 and 100 mg/kg caffeine,
and probes were implanted in the left brain hemisphere, either in the
shell of the NAc [coordinates with respect to bregma: anterior (A), ⫹2.3;
lateral (L), 1.0; ventral (V), 7.8 from the dura] or in the core of the NAc
(A, ⫹1.6; L, 1.8; V, 7.6). The experiments were performed on freely
moving rats 24 hr after the probe implantation. A solution (in mM) of 147
Ringer's, 4 KCl, and 2.2 CaCl2 was pumped through the dialysis probe at
a constant rate of 0.4 l/min from immediately after implantation until
the beginning of the microdialysis experiment, when it was switched to 1
l/min until the end of the experiment. Samples were collected at 20 min
Figure 2. Extracellular concentrations of dopamine (DA) and glutamate
intervals and split into two 10 l fractions. One 10 l fraction was assayed
(Glu) in the shell of the NAc after intraperitoneal administration of saline
for dopamine content and the other was assayed for glutamate content,
or caffeine [3 (caff 3), 10 (caff 10), 30 (caff 30), or 100 (caff 100) mg/kg].
using HPLC systems with electrochemical (for dopamine) and fluores-
The results represent means ⫾ SEM of the percentage of basal values of
cence (for glutamate) detection, as described in detail previously (You et
the extracellular concentrations of dopamine and glutamate (n ⫽ 6–8 per
al., 1994; Pontieri et al., 1995). At the end of the experiment, rats were
group). Basal values were the means of three values before drug admin-
killed with an overdose of Equithesin and methylene blue was perfused
istration. Caffeine at doses of 10 and 30 mg/kg but not at doses of 3 and
through the probe. The brain was removed and placed in a 10% form-
100 mg/kg significantly increased the extracellular levels of dopamine and
aldehyde solution, and coronal sections were cut to verify the probe
glutamate (Student's paired t test; only significant results of pretreatment
location. The statistical analysis used was the "summary measures"
vs post-treatment are shown).
method (Matthews et al., 1990), using the mean of the three values
previous to drug administration and the mean of the six values subse-
quent to drug administration per animal as the summary measures.
concentrations of dopamine (up by ⬃100%) and glutamate (up by
Pretreatment and post-treatment dopamine values obtained from the
⬃50%) in the shell of the NAc (Fig. 2). These effects of caffeine
shell and core of the NAc in animals treated with 30 mg/kg caffeine were
were dose- and region-specific. Thus, neither 3 nor 100 mg/kg of
analyzed by repeated-measures ANOVA to assess treatment and brain
area effects. Pretreatment versus post-treatment values were compared
caffeine significantly modified dopamine or glutamate extracellu-
using Student's paired t test to analyze treatment effects; p values shown
lar levels in the shell of the NAc (Fig. 2); in the core of the NAc,
in the figures refer in all cases to these differences.
30 mg/kg caffeine induced a small but significant increase in
dopamine levels (⬃25%) and no significant changes in glutamate
levels (Fig. 3). This effect was significantly different from that
Determination of the motor-activating doses
produced by 30 mg/kg caffeine in the shell of the NAc (interac-
of caffeine
tion between area and treatment factors: p ⬍ 0.01 by repeated-
A dose–response study of the motor activity induced by intra-
measures ANOVA). Basal extracellular levels of dopamine and
peritoneal administration of caffeine was performed in male
glutamate were 4.2 ⫾ 0.3 nM (n ⫽ 45) and 3.2 ⫾ 0.2 M (n ⫽ 52),
Sprague Dawley rats to establish behaviorally relevant doses for
respectively, in the shell of the NAc and 4.6 ⫾ 0.6 nM (n ⫽ 9) and
microdialysis studies. As reported previously (Daly and Fred-
3.4 ⫾ 0.6 M (n ⫽ 10), respectively, in the core of the NAc.
holm, 1998), the motor-activation dose–response curve produced
by caffeine had an inverted U shape, with 3 and 100 mg/kg doses
Effects of caffeine on dopamine and glutamate release
being ineffective and 10 and 30 mg/kg producing a similar max-
reproduced by a selective adenosine A1 receptor
antagonist
imal increase in motor activity (Fig. 1).
Because caffeine is a nonselective adenosine receptor antagonist,
Caffeine induces a preferential release of dopamine
the selective adenosine A1 receptor antagonist CPT and the
and glutamate in the shell of the NAc
selective adenosine A2A receptor antagonist SCH 58261 were
In subsequent microdialysis experiments, 10 and 30 mg/kg doses
used to investigate the adenosine receptors involved. Low motor-
of caffeine induced similar significant increases in extracellular
activating doses of both CPT (4.8 mg/kg) and SCH 58261 (2
Solinas et al. • Caffeine-Induced Dopamine and Glutamate Release
J. Neurosci., August 1, 2002, 22(15):6321–6324 6323
Figure 3. Extracellular concentrations of dopamine (DA) and glutamate
Figure 4. Extracellular concentrations of dopamine (DA) and glutamate
(Glu) in the shell and core of the NAc after intraperitoneal administration
(Glu) in the shell of the NAc after intraperitoneal administration of the
of caffeine [30 mg/kg (caff 30)]. The results represent means ⫾ SEM of
adenosine A1 receptor antagonist CPT (4.8 mg/kg) and the A2A recep-
the percentage of basal values of the extracellular concentrations of
tor antagonist SCH 58261 (2 mg/kg). The results represent means ⫾ SEM
dopamine and glutamate (n ⫽ 6–8 per group). Basal values were the
of the percentage of basal values of the extracellular concentrations of
means of three values before drug administration. Caffeine (30 mg/kg)
dopamine and glutamate (n ⫽ 6–7 per group). Basal values were the
produced a significant increase in the extracellular concentration of
means of three values before drug administration. CPT but not SCH
dopamine, but not of glutamate, in the core of the NAc (Student's paired
58261 produced a significant increase in the extracellular concentrations
t test; only significant results of pretreatment vs post-treatment are
of dopamine and glutamate (Student's paired t test; only significant results
shown). This effect was significantly different from that produced by 30
of pretreatment vs post-treatment are shown).
mg/kg caffeine in the shell of the NAc (interaction between area and
treatment factors: p ⬍ 0.01 by repeated-measures ANOVA).
rats blocked the locomotor activation induced by amphetamines
but failed to block the locomotor activation induced by caffeine.
mg/kg) were used (Popoli et al., 1998). It was found that CPT but
Based on these results, the authors suggested that caffeine in-
not SCH 58261 significantly increased extracellular levels of do-
duces locomotor activity by acting independently of presynaptic
pamine and glutamate (⬃100% in both cases) in the shell of the
terminals in the mesolimbic dopaminergic system (Joyce and
NAc (Fig. 4).
Koob, 1981; Swerdlow et al., 1986). However, in view of the
demonstrated resistance to 6-hydroxydopamine-induced dopa-
mine denervation in the shell versus the core of the NAc
Dopamine release in either the core or the shell of the NAc has
(Meredith et al., 1995; Boye et al., 2001), those studies cannot rule
been suggested to be causally related to the locomotor stimulant
out a preferential role of dopamine release in the shell of the NAc
effects of psychostimulants such as amphetamine (Parkinson et
on the motor effects induced by caffeine. Nevertheless, it must be
al., 1999; Boye et al., 2001), whereas preferential release of
pointed out that other striatal regions can also be involved,
dopamine in the shell of the accumbens has been suggested
because a significant although less pronounced effect of caffeine
to be causally related to the rewarding effects of psychostimulants
on dopamine release was also observed in the core of the NAc
(Di Chiara and Imperato, 1988). The close correlations between
(see the introductory remarks; Morgan and Vestal, 1989). Sur-
the motor-activating effects and the previously described
prisingly, the highest dose of caffeine (100 mg/kg) did not pro-
discriminative-stimulus effects of caffeine (Mumford and Holtz-
duce any effect on extracellular dopamine or glutamate levels in
man, 1991) and the present microdialysis data are consistent with
the shell of the NAc. Additional studies are needed to clarify the
the possibility that the preferential release of dopamine and
mechanisms involved in this lack of effect. However, high doses of
glutamate in the shell of the NAc may also be involved in the
caffeine are known to exert effects through mechanisms other
psychostimulant effects of caffeine. Our hypothesis might seem to
than adenosine receptor antagonism (e.g., phosphodiesterase in-
be in conflict with the studies by Joyce and Koob (1981), who
hibition and release of intracellular calcium) (Daly and Fred-
found that 6-hydroxydopamine lesions in the region of the NAc of
holm, 1998).
6324 J. Neurosci., August 1, 2002, 22(15):6321–6324
Solinas et al. • Caffeine-Induced Dopamine and Glutamate Release
The results obtained with the selective adenosine A1 and A2A
Daly JW, Fredholm BB (1998) An atypical drug of dependence. Drug
receptor antagonists indicate that the effects of the lower 10 and
Alcohol Depend 51:199–206.
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially
30 mg/kg doses of caffeine on dopamine and glutamate release
increase synaptic dopamine concentrations in the mesolimbic system of
are related to adenosine A1 receptor antagonism. Thus, although
freely moving rats. Proc Natl Acad Sci USA 85:5274–5278.
blockade of adenosine A2A receptors is currently believed to be
Ferre´ S, Fredholm BB, Morelli M, Popoli P, Fuxe K (1997) Adenosine-
dopamine receptor-receptor interactions as an integrative mechanism
the main mechanism responsible for the behavioral-activating
in the basal ganglia. Trends Neurosci 20:482–487.
(psychostimulant) effects of caffeine (Daly and Fredholm, 1998),
Flagmeyer I, Haas HL, Stevens DR (1997) Adenosine A1 receptor-
as suggested previously (Snyder et al., 1981; Nikodijevic et al.,
mediated depression of corticostriatal and thalamostriatal glutamater-
gic synaptic potentials in vitro. Brain Res 778:178–185.
1991; Kaplan et al., 1992; Popoli et al., 1998), blockade of aden-
Golembiowska K, Zylewska A (1997) Adenosine receptors: the role in
osine A1 receptors also may play a relevant role. At the dose used
modulation of dopamine and glutamate release in the rat striatum. Pol
in the present study, the A2A receptor antagonist had been shown
J Pharmacol 49:317–322.
Joyce EM, Koob GF (1981) Amphetamine-, scopolamine-, and caffeine-
previously to induce pronounced motor activation (Popoli et al.,
induced locomotor activity following 6-hydroxydopamine lesions of the
1998). This rules out the possibility that the motor response is
mesolimbic dopamine system. Psychopharmacology 73:311–313.
responsible for the dopamine release in the NAc induced by
Kaplan GB, Greenblatt DJ, Kent MA, Cotreau MM, Arcelin G, Shader
RI (1992) Caffeine-induced behavioral stimulation is dose-dependent
caffeine or the A1 receptor antagonist. The most probable local-
and associated with A1 adenosine receptor occupancy. Neuropsychop-
ization of the adenosine A1 receptors that modulate caffeine-
induced elevations in extracellular dopamine and glutamate lev-
Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of
serial measurements in medical research. BMJ 300:230–235.
els is in the terminals of dopaminergic and glutamatergic afferents
Meredith GE, Ypma P, Zahm DS (1995) Effects of dopamine depletion
to the NAc. In fact, there is morphological and functional evi-
on the morphology of medium spiny neurons in the shell and core of the
dence for this presynaptic localization of adenosine A1 receptors
rat nucleus accumbens. J Neurosci 15:3808–3820.
Morari M, Marti M, Sbrenna S, Fuxe K, Bianchi C, Beani L (1998)
(Wood et al., 1989; Okada et al., 1996; Flagmeyer et al., 1997;
Reciprocal dopamine-glutamate modulation of release in the basal
Golembiowska and Zylewska, 1997). Also, microdialysis studies
ganglia. Neurochem Int 33:383–397.
have shown previously that the striatal perfusion of A1 receptor
Morgan ME, Vestal RE (1989) Methylxanthine effects on caudate do-
pamine release as measured by in vivo electrochemistry. Life Sci
agonists and antagonists significantly modifies (decreases and
increases, respectively) the striatal extracellular concentrations of
Mumford GK, Holtzman SG (1991) Qualitative differences in the dis-
dopamine and glutamate (Okada et al., 1996; Golembiowska and
criminative stimulus effects of low and high doses of caffeine in rats.
J Pharmacol Exp Ther 258:857–865.
Zylewska, 1997). Finally, the increase in the extracellular levels of
Nikodijevic O, Sarges R, Daly JW, Jacobson KA (1991) Behavioral
dopamine induced by caffeine and the A1 receptor antagonist
effects of A1- and A2-selective adenosine agonists and antagonists:
could be related to their effects on extracellular glutamate, in
evidence for synergism and antagonism. J Pharmacol Exp Ther
view of the evidence for a facilitatory role of glutamate on striatal
Okada M, Mizuno K, Kaneko S (1996) Adenosine A1 and A2 receptors
dopamine release (Morari et al., 1998).
modulate extracellular dopamine levels in rat striatum. Neurosci Lett
The region-dependent effects of caffeine in the NAc are similar
Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ (1999)
to those produced by other psychostimulants and addictive drugs,
Dissociation in effects of lesions of the nucleus accumbens core and
such as amphetamine, cocaine, morphine, heroin, nicotine or
shell on appetitive pavlovian approach behavior and the potentiation of
conditioned reinforcement and locomotor activity by
9-tetrahydrocannabinol (⌬9-THC), all of which preferentially
J Neurosci 19:2401–2411.
increase extracellular levels of dopamine in the shell of the NAc
Pontieri FE, Tanda G, Di Chiara G (1995) Intravenous cocaine, mor-
(Pontieri et al., 1995). Although the degree of increase in extra-
phine, and amphetamine preferentially increase extracellular dopamine
in the "shell" as compared with the "core" of the rat nucleus accum-
cellular dopamine levels induced by caffeine is lower than that
bens. Proc Natl Acad Sci USA 92:12304–12308.
induced by amphetamine and cocaine, it is in the same range as
Popoli P, Reggio R, Pezzola A, Fuxe K, Ferre´ S (1998) Adenosine A1
increases induced by the systemic administration of nicotine (Di
and A2A receptor antagonists stimulate motor activity: evidence for an
increased effectiveness in aged rats. Neurosci Lett 251:201–204.
Chiara and Imperato, 1988), ⌬9-THC (Chen et al., 1991), mor-
Reid MS, Hsu K, Berger SP (1997) Cocaine and amphetamine prefer-
phine (Di Chiara and Imperato, 1988; Pontieri et al., 1995), or
entially stimulate glutamate release in the limbic system: studies on the
ethanol (Di Chiara and Imperato, 1988). Because these neuro-
involvement of dopamine. Synapse 27:95–105.
Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW (1981) Adenosine
chemical changes are often considered central to the development
receptors and behavioural actions of methylxanthines. Proc Natl Acad
of drug dependence, they could contribute to the widespread
Sci USA 78:3260–3264.
consumption of caffeine-containing beverages.
Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF (1986) The neural
substrates for the motor-activating properties of psychostimulants: a
review of recent findings. Pharmacol Biochem Behav 25:233–248.
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of ad-
Boye SM, Grant RJ, Clarke PB (2001) Disruption of dopaminergic neu-
diction. Psychol Rev 94:469–492.
rotransmission in nucleus accumbens core inhibits the locomotor stim-
Wood PL, Kim HS, Boyar WC, Hutchison A (1989) Inhibition of nigro-
ulant effects of nicotine and D-amphetamine in rats. Neuropharmacol-
striatal release of dopamine in the rat by adenosine receptor agonists:
ogy 40:792–805.
A1 receptor mediation. Neuropharmacology 28:21–25.
Chen J, Paredes W, Lowinson JH, Gardner EL (1991) Strain-specific
You ZB, Herrera-Marschitz M, Brodin E, Meana JJ, Morino P, Hokfelt
facilitation of dopamine efflux by ␦9-tetrahydrocannabinol in the nu-
T, Silveira R, Goiny M, Ungerstedt U (1994) On the origin of striatal
cleus accumbens of rat: an in vivo microdialysis study. Neurosci Lett
cholecystokinin release: studies with in vivo microdialysis. J Neuro-
chem 62:76–85.
Source: http://www.healthislife.org/uploads/6/7/2/9/6729888/caffiene_increases_na_da_by_blocking_adenosine.pdf
Josep Tabernero, MD, PhDModerator Case ForummCRC – Case Study 1 Fortunato Ciardiello, MD, PhDSecond University of NaplesNaples, Italy Case Study 1 Fortunato Ciardiello, MD, PhD Meet Sandra Sandra is a 69-year-old woman with rectal cancer. At the time of diagnosis in early 2010, her only medical condition was hypertension.
Lichen sclerosus. DermNet NZ 12/24/2007 07:19 PM Authoritative facts about the skin from the New Zealand Dermatological Society Incorporated. Home Immunological disorders Lichen sclerosus is chronic skin disorder that most often affects the genital and perianal areas. It usually persistsfor years, and can cause permanent scarring. There is no known cure, although most people are substantiallyimproved and quite comfortable with treatment.





